Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI)
- PMID: 19111009
- PMCID: PMC2724718
- DOI: 10.1002/mrm.21875
Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI)
Abstract
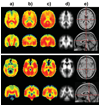
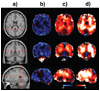
Distributions of proton MR-detected metabolites have been mapped throughout the brain in a group of normal subjects using a volumetric MR spectroscopic imaging (MRSI) acquisition with an interleaved water reference. Data were processed with intensity and spatial normalization to enable voxel-based analysis methods to be applied across a group of subjects. Results demonstrate significant regional, tissue, and gender-dependent variations of brain metabolite concentrations, and variations of these distributions with normal aging. The greatest alteration of metabolites with age was observed for white-matter choline and creatine. An example of the utility of the normative metabolic reference information is then demonstrated for analysis of data acquired from a subject who suffered a traumatic brain injury. This study demonstrates the ability to obtain proton spectra from a wide region of the brain and to apply fully automated processing methods. The resultant data provide a normative reference for subsequent utilization for studies of brain injury and disease.
Figures



References
-
- Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58:2049–2056. - PubMed
-
- McLean MA, Woermann FG, Barker GJ, Duncan JS. Quantitative analysis of short echo time 1H-MRSI of cerebral gray and white matter. Magn Reson Med. 2000;44:401–411. - PubMed
-
- Harada M, Miyoshi H, Otsuka H, Nishitani H, Uno M. Multivariate analysis of regional metabolic differences in normal ageing on localised quantitative proton MR spectroscopy. Neuroradiology. 2001;43:448–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

