Direct and continuous assay for prolyl 4-hydroxylase
- PMID: 19111518
- PMCID: PMC2643311
- DOI: 10.1016/j.ab.2008.11.046
Direct and continuous assay for prolyl 4-hydroxylase
Abstract
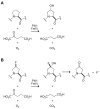
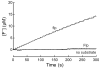
Prolyl 4-hydroxylase (P4H) is a nonheme iron dioxygenase that catalyzes the posttranslational hydroxylation of (2S)-proline (Pro) residues in protocollagen strands. The resulting (2S,4R)-4-hydroxyproline (Hyp) residues are essential for the folding, secretion, and stability of the collagen triple helix. P4H uses alpha-ketoglutarate and O2 as cosubstrates, and forms succinate and CO2 as well as Hyp. Described herein is the first assay for P4H that continuously and directly detects turnover of the proline-containing substrate. This assay is based on (2S,4S)-4-fluoroproline (flp), a proline analogue that is transformed into (2S)-4-ketoproline (Kep) and inorganic fluoride by P4H. The fluoride ion, and thus turnover by P4H, is detected by a fluoride ion-selective electrode. Using this assay, steady-state kinetic parameters for the human P4H-catalyzed turnover of a flp-containing peptide were determined and found to be comparable to those obtained with a discontinuous HPLC-based assay. In addition, this assay can be used to characterize P4H variants, as demonstrated by a comparison of catalysis by D414A P4H and the wild-type enzyme. Finally, the use of the assay to identify small-molecule inhibitors of P4H was verified by an analysis of catalysis in the presence of 2,4-pyridine dicarboxylate, an analogue of alpha-ketoglutarate. Thus, the assay described herein could facilitate biochemical analyses of this essential enzyme.
Figures



References
-
- Prockop DJ, Kivirikko KI. Collagens: Molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. - PubMed
-
- Berg RA, Prockop DJ. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple helix of collagen. Biochem Biophys Res Comm. 1973;52:115–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

