Mechanisms mediating androgen receptor reactivation after castration
- PMID: 19111796
- PMCID: PMC3245883
- DOI: 10.1016/j.urolonc.2008.03.021
Mechanisms mediating androgen receptor reactivation after castration
Abstract
Androgen deprivation is still the standard systemic therapy for metastatic prostate cancer (PCa), but patients invariably relapse with a more aggressive form of PCa termed hormone refractory, androgen independent, or castration resistant PCa (CRPC). Significantly, the androgen receptor (AR) is expressed at high levels in most cases of CRPC, and these tumors resume their expression of multiple AR-regulated genes, indicating that AR transcriptional activity becomes reactivated at this stage of the disease. The molecular basis for this AR reactivation remains unclear, but possible mechanisms include increased AR expression, AR mutations that enhance activation by weak androgens and AR antagonists, increased expression of transcriptional coactivator proteins, and activation of signal transduction pathways that can enhance AR responses to low levels of androgens. Recent data indicate that CRPC cells may also carry out intracellular synthesis of testosterone and DHT from weak adrenal androgens and may be able to synthesize androgens from cholesterol. These mechanisms that appear to contribute to AR reactivation after castration are further outlined in this review.
Figures

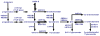
References
-
- Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–20222. - PubMed
-
- Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. - PubMed
-
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. - PubMed
-
- Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

