Chronic hypoxia enhances 15-lipoxygenase-mediated vasorelaxation in rabbit arteries
- PMID: 19112096
- PMCID: PMC2660232
- DOI: 10.1152/ajpheart.00777.2008
Chronic hypoxia enhances 15-lipoxygenase-mediated vasorelaxation in rabbit arteries
Retraction in
-
Retraction.Am J Physiol Heart Circ Physiol. 2014 Aug 15;307(4):H633. doi: 10.1152/ajpheart.zh4-1249-retr.2014. Am J Physiol Heart Circ Physiol. 2014. PMID: 25128501 Free PMC article. No abstract available.
Abstract
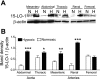
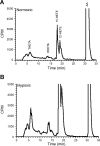
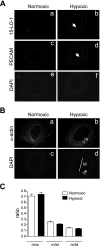
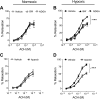
15-Lipoxygenase (15-LO-1) metabolizes arachidonic acid (AA) to 11,12,15-trihydroxyeicosatrienoic acids (THETAs) and 15-hydroxy-11,12-epoxyeicosatrienoic acids (HEETA) that dilate rabbit arteries. Increased endothelial 15-LO-1 expression enhances arterial relaxations to agonists. We tested the effect of hypoxia on 15-LO-1 expression, THETA and HEETA synthesis, and relaxations in rabbit arteries. The incubation of rabbit aortic endothelial cells and isolated aortas in 0.7% O(2) increased 15-LO-1 expression. Rabbits were housed in a hypoxic atmosphere of 12% O(2) for 5 days. 15-LO-1 expression increased in the endothelium of the arteries of rabbits in 12% O(2) compared with room air. THETA and HEETA synthesis was also enhanced in aortas and mesenteric arteries. AA hyperpolarized the smooth muscle cells in indomethacin- and phenylephrine-treated mesenteric arteries of hypoxic rabbits from -29.4 +/- 1 to -50.1 +/- 3 mV. The hyperpolarization to AA was less in arteries of normoxic rabbits (from -26.0 +/- 2 to -37 +/- 2 mV). This AA-induced hyperpolarization was inhibited by the 15-LO inhibitor BW-755C. Nitric oxide and prostaglandin-independent maximum relaxations to acetylcholine (79.7 +/- 2%) and AA (38.3 +/- 4%) were enhanced in mesenteric arteries from hypoxic rabbits compared with the normoxic rabbits (49.7 +/- 6% and 19.9 +/- 2%, respectively). These relaxations were inhibited by BW-755C and nordihydroguaiaretic acid. Therefore, hypoxia increased the relaxations to agonists in the rabbit mesenteric arteries by enhancing endothelial 15-LO-1 expression and synthesis of the hyperpolarizing factors THETA and HEETA.
Figures
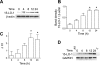



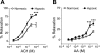

Comment in
-
Findings of research misconduct.NIH Guide Grants Contracts (Bethesda). 2013 Oct 25:NOT-OD-14-010. NIH Guide Grants Contracts (Bethesda). 2013. PMID: 24163871 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2013 Oct 2;78(191):60873-60874. Fed Regist. 2013. PMID: 27737231 Free PMC article. No abstract available.
References
-
- Aggarwal NT, Chawengsub Y, Gauthier KM, Viita H, Yla-Herttuala S, Campbell WB. Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits. Hypertension 51: 246–251, 2008. - PubMed
-
- Aggarwal NT, Holmes BB, Cui L, Viita H, Yla-Herttuala S, Campbell WB. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: role in arachidonic acid-induced relaxation. Am J Physiol Heart Circ Physiol 292: H1033–H1041, 2007. - PubMed
-
- Bajpai AK, Blaskova E, Pakala SB, Zhao T, Glasgow WC, Penn JS, Johnson DA, Rao GN. 15(S)-HETE production in human retinal microvascular endothelial cells by hypoxia: novel role for MEK1 in 15(S)-HETE induced angiogenesis. Invest Ophthalmol Vis Sci 48: 4930–4938, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

