Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates
- PMID: 19112491
- PMCID: PMC2597720
- DOI: 10.1371/journal.pgen.1000317
Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates
Abstract
In many mammalian neurons, dense clusters of ion channels at the axonal initial segment and nodes of Ranvier underlie action potential generation and rapid conduction. Axonal clustering of mammalian voltage-gated sodium and KCNQ (Kv7) potassium channels is based on linkage to the actin-spectrin cytoskeleton, which is mediated by the adaptor protein ankyrin-G. We identified key steps in the evolution of this axonal channel clustering. The anchor motif for sodium channel clustering evolved early in the chordate lineage before the divergence of the wormlike cephalochordate, amphioxus. Axons of the lamprey, a very primitive vertebrate, exhibited some invertebrate features (lack of myelin, use of giant diameter to hasten conduction), but possessed narrow initial segments bearing sodium channel clusters like in more recently evolved vertebrates. The KCNQ potassium channel anchor motif evolved after the divergence of lampreys from other vertebrates, in a common ancestor of shark and humans. Thus, clustering of voltage-gated sodium channels was a pivotal early innovation of the chordates. Sodium channel clusters at the axon initial segment serving the generation of action potentials evolved long before the node of Ranvier. KCNQ channels acquired anchors allowing their integration into pre-existing sodium channel complexes at about the same time that ancient vertebrates acquired myelin, saltatory conduction, and hinged jaws. The early chordate refinements in action potential mechanisms we have elucidated appear essential to the complex neural signaling, active behavior, and evolutionary success of vertebrates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


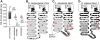



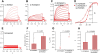

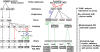
Comment in
-
Converging on the origins of axonal ion channel clustering.PLoS Genet. 2009 Jan;5(1):e1000340. doi: 10.1371/journal.pgen.1000340. Epub 2009 Jan 16. PLoS Genet. 2009. PMID: 19148277 Free PMC article. No abstract available.
References
-
- Hille B. Ionic channels of excitable membranes. Sunderland, Mass.: Sinauer; 2001.
-
- Schweigreiter R, Roots BI, Bandtlow CE, Gould RM. Understanding myelination through studying its evolution. Int Rev Neurobiol. 2006;73:219–273. - PubMed
-
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–35. - PubMed
-
- Ramon y Cajal S. Texture of the nervous system of man and the vertebrates. New York: SpringerWein; 1907. Pasic P, Pasic T, translators and editors. 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

