Topographical characterization of cone photoreceptors and the area centralis of the canine retina
- PMID: 19112529
- PMCID: PMC2610288
Topographical characterization of cone photoreceptors and the area centralis of the canine retina
Abstract
Purpose: The canine is an important large animal model of human retinal genetic disorders. Studies of ganglion cell distribution in the canine retina have identified a visual streak of high density superior to the optic disc with a temporal area of peak density known as the area centralis. The topography of cone photoreceptors in the canine retina has not been characterized in detail, and in contrast to the macula in humans, the position of the area centralis in dogs is not apparent on clinical funduscopic examination. The purpose of this study was to define the location of the area centralis in the dog and to characterize in detail the topography of rod and cone photoreceptors within the area centralis. This will facilitate the investigation and treatment of retinal disease in the canine.
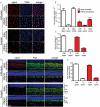
Methods: We used peanut agglutinin, which labels cone matrix sheaths and antibodies against long/medium wavelength (L/M)- and short wavelength (S)-cone opsins, to stain retinal cryosections and flatmounts from beagle dogs. Retinas were imaged using differential interference contrast imaging, fluorescence, and confocal microscopy. Within the area centralis, rod and cone size and density were quantified, and the proportion of cones expressing each cone opsin subtype was calculated. Using a grid pattern of sampling in 9 retinal flatmounts, we investigated the distribution of cones throughout the retina to predict the location of the area centralis.
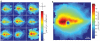
Results: We identified the area centralis as the site of maximal density of rod and cone photoreceptor cells, which have a smaller inner segment cross-sectional area in this region. L/M opsin was expressed by the majority of cones in the retina, both within the area centralis and in the peripheral retina. Using the mean of cone density distribution from 9 retinas, we calculated that the area centralis is likely to be centered at a point 1.5 mm temporal and 0.6 mm superior to the optic disc. For clinical funduscopic examination, this represents 1.2 disc diameters temporal and 0.4 disc diameters superior to the optic disc.
Conclusions: We have described the distribution of rods and cone subtypes within the canine retina and calculated a predictable location for the area centralis. These findings will facilitate the characterization and treatment of cone photoreceptor dystrophies in the dog.
Figures

References
-
- Ropstad EO, Narfstrom K, Lingaas F, Wiik C, Bruun A, Bjerkas E. Functional and Structural Changes in the Retina of Wire-Haired Dachshunds with Early-Onset Cone-Rod Dystrophy. Invest Ophthalmol Vis Sci. 2008;49:1106–15. - PubMed
-
- Mellersh CS, Boursnell ME, Pettitt L, Ryder EJ, Holmes NG, Grafham D, Forman OP, Sampson J, Barnett KC, Blanton S, Binns MM, Vaudin M. Canine RPGRIP1 mutation establishes cone-rod dystrophy in miniature longhaired dachshunds as a homologue of human Leber congenital amaurosis. Genomics. 2006;88:293–301. - PubMed
-
- Zangerl B, Goldstein O, Philp AR, Lindauer SJ, Pearce-Kelling SE, Mullins RF, Graphodatsky AS, Ripoll D, Felix JS, Stone EM, Acland GM, Aguirre GD. Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics. 2006;88:551–63. - PMC - PubMed
-
- Kijas JW, Miller BJ, Pearce-Kelling SE, Aguirre GD, Acland GM. Canine Models of Ocular Disease: Outcross Breedings Define a Dominant Disorder Present in the English Mastiff and Bull Mastiff Dog Breeds. J Hered. 2003;94:27–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
