Design and Applications of Bifunctional Small Molecules: Why Two Heads Are Better Than One
- PMID: 19112665
- PMCID: PMC2925120
- DOI: 10.1021/cb8001792
Design and Applications of Bifunctional Small Molecules: Why Two Heads Are Better Than One
Abstract
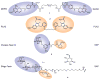
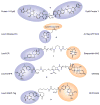
Induction of protein--protein interactions is a daunting challenge, but recent studies show promise for small molecules that specifically bring two or more protein molecules together for enhanced or novel biological effect. The first such bifunctional molecules were the rapamycin- and FK506-based "chemical inducers of dimerization", but the field has since expanded with new molecules and new applications in chemical genetics and cell biology. Examples include coumermycin-mediated gyrase B dimerization, proteolysis targeting chimeric molecules (PROTACs), drug hybrids, and strategies for exploiting multivalency in toxin binding and antibody recruitment. This Review discusses these and other advances in the design and use of bifunctional small molecules and potential strategies for future systems.
Figures






References
-
- Chene P. Drugs targeting protein-protein interactions. Chem Med Chem. 2006;1:400–411. - PubMed
-
- Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. - PubMed
-
- Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–317. - PubMed
-
- Yin H, Hamilton AD. Strategies for targeting protein-protein interactions with synthetic agents. Angew Chem Int Ed. 2005;44:4130–4163. - PubMed
-
- Mossessova E, Corpina RA, Goldberg J. Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell. 2003;12:1403–1411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

