Induced association of mu opioid (MOP) and type 2 cholecystokinin (CCK2) receptors by novel bivalent ligands
- PMID: 19113864
- PMCID: PMC2650857
- DOI: 10.1021/jm800174p
Induced association of mu opioid (MOP) and type 2 cholecystokinin (CCK2) receptors by novel bivalent ligands
Abstract
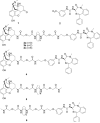
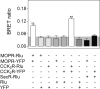
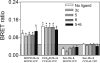
Both mu-opioid (MOP) and type 2 cholecystokinin (CCK2) receptors are present in areas of the central nervous system that are involved in modulation of pain processing. We conducted bioluminescence resonance energy transfer (BRET) studies on COS cells coexpressing MOP and CCK2 receptors to determine whether receptor heterodimerization is involved in such modulation. These studies revealed the absence of constitutive or monovalent ligand-induced heterodimerization. Heterodimerization of MOP and CCK2 receptors therefore is unlikely to be responsible for the opposing effects between morphine and CCK in the CNS. However, association was induced, as indicated by a positive BRET signal, on exposure of the cells to bivalent ligands containing mu-opioid agonist and CCK2 receptor antagonist pharmacophores linked through spacers containing 16-22 atoms but not with a shorter (9-atom) spacer. These studies demonstrate for the first time that an appropriately designed bivalent ligand is capable of inducing association of G-protein-coupled receptors. The finding that opioid tolerance studies with these ligands in mice showed no correlation with the BRET data is consistent with the absence of association of MOP and CCK2 receptors in vivo.
Figures
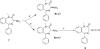
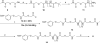

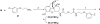
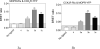
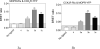

Similar articles
-
Modulation of cell surface expression of nonactivated cholecystokinin receptors using bivalent ligand-induced internalization.J Med Chem. 2010 Apr 8;53(7):2836-42. doi: 10.1021/jm100135g. J Med Chem. 2010. PMID: 20235611 Free PMC article.
-
Estrogen and CCK1 receptor modification of mu-opioid receptor binding in the cortex of female rats.Brain Res. 2006 Feb 16;1073-1074:316-20. doi: 10.1016/j.brainres.2005.12.023. Epub 2006 Feb 10. Brain Res. 2006. PMID: 16472782
-
Bivalent ligands that target μ opioid (MOP) and cannabinoid1 (CB1) receptors are potent analgesics devoid of tolerance.J Med Chem. 2013 Jul 11;56(13):5505-13. doi: 10.1021/jm4005219. Epub 2013 Jun 20. J Med Chem. 2013. PMID: 23734559 Free PMC article.
-
Heteromers of μ-δ opioid receptors: new pharmacology and novel therapeutic possibilities.Br J Pharmacol. 2015 Jan;172(2):375-87. doi: 10.1111/bph.12663. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24571499 Free PMC article. Review.
-
Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile.Br J Anaesth. 2009 Jul;103(1):38-49. doi: 10.1093/bja/aep129. Epub 2009 May 27. Br J Anaesth. 2009. PMID: 19474215 Review.
Cited by
-
Toward the Development of Bivalent Ligand Probes of Cannabinoid CB1 and Orexin OX1 Receptor Heterodimers.ACS Med Chem Lett. 2014 Mar 25;5(6):634-8. doi: 10.1021/ml4004759. eCollection 2014 Jun 12. ACS Med Chem Lett. 2014. PMID: 24944734 Free PMC article.
-
Designing bifunctional NOP receptor-mu opioid receptor ligands from NOP receptor-selective scaffolds. Part I.Bioorg Med Chem Lett. 2013 Jun 1;23(11):3308-13. doi: 10.1016/j.bmcl.2013.03.101. Epub 2013 Apr 4. Bioorg Med Chem Lett. 2013. PMID: 23623415 Free PMC article.
-
Developing a Biased Unmatched Bivalent Ligand (BUmBL) Design Strategy to Target the GPCR Homodimer Allosteric Signaling (cAMP over β-Arrestin 2 Recruitment) Within the Melanocortin Receptors.J Med Chem. 2019 Jan 10;62(1):144-158. doi: 10.1021/acs.jmedchem.8b00238. Epub 2018 May 9. J Med Chem. 2019. PMID: 29669202 Free PMC article.
-
Bivalent Ligand Aiming Putative Mu Opioid Receptor and Chemokine Receptor CXCR4 Dimers in Opioid Enhanced HIV-1 Entry.ACS Med Chem Lett. 2020 Sep 13;11(11):2318-2324. doi: 10.1021/acsmedchemlett.0c00444. eCollection 2020 Nov 12. ACS Med Chem Lett. 2020. PMID: 33214847 Free PMC article.
-
Novel bivalent ligands carrying potential antinociceptive effects by targeting putative mu opioid receptor and chemokine receptor CXCR4 heterodimers.Bioorg Chem. 2022 Mar;120:105641. doi: 10.1016/j.bioorg.2022.105641. Epub 2022 Jan 24. Bioorg Chem. 2022. PMID: 35093692 Free PMC article.
References
-
- IUPHAR RECEPTOR DATABASE, Opioid receptors, http://uphardb.org/PRODGPCR.
-
- Gutstein HB, Akil H. The pharmacological Basis of Therapeutics(11th Ed. The McGraw-Hill Companies, Inc.; USA: 2006. Goodman and Gillman's. Chapter 21 opioid analgesics.
-
- Saito A, Goldfine ID, Williams JA. Characterization of receptors for cholecystokinin and related peptides in mouse cerebral cortex. J. Neurochem. 1981;37:483–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

