Genetic evidence for the importance of protein acetylation and protein deacetylation in the halophilic archaeon Haloferax volcanii
- PMID: 19114494
- PMCID: PMC2648188
- DOI: 10.1128/JB.01252-08
Genetic evidence for the importance of protein acetylation and protein deacetylation in the halophilic archaeon Haloferax volcanii
Abstract
Protein acetylation and deacetylation reactions are involved in many regulatory processes in eukaryotes. Recently, it was found that similar processes occur in bacteria and archaea. Sequence analysis of the genome of the haloarchaeon Haloferax volcanii led to the identification of three putative protein acetyltransferases belonging to the Gcn5 family, Pat1, Pat2, and Elp3, and two deacetylases, Sir2 and HdaI. Intriguingly, the gene that encodes HdaI shares an operon with an archaeal histone homolog. We performed gene knockouts to determine whether the genes encoding these putative acetyltransferases and deacetylases are essential. A sir2 deletion mutant was able to grow normally, whereas an hdaI deletion mutant was nonviable. The latter is consistent with the finding that trichostatin A, a specific inhibitor of HdaI, inhibits cell growth in a concentration-dependent manner. We also showed that each of the acetyltransferases by itself is dispensable for growth but that deletion of both pat2 and elp3 could not be achieved. The corresponding genes are therefore "synthetic lethals," and the protein acetyltransferases probably have a common and essential substrate.
Figures


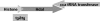


References
-
- Aivaliotis, M., K. Gevaert, M. Falb, A. Tebbe, K. Konstantinidis, B. Bisle, C. Klein, L. Martens, A. Staes, E. Timmerman, J. Van Damme, F. Siedler, F. Pfeiffer, J. Vandekerckhove, and D. Oesterhelt. 2007. Large-scale identification of N-terminal peptides in the halophilic archaea Halobacterium salinarum and Natronomonas pharaonis. J. Proteome Res. 62195-2204. - PubMed
-
- Allers, T., and M. Mevarech. 2005. Archaeal genetics—the third way. Nat. Rev. Genet. 658-73. - PubMed
-
- Avalos, J. L., J. D. Boeke, and C. Wolberger. 2004. Structural basis for the mechanism and regulation of Sir2 enzymes. Mol. Cell 13639-648. - PubMed
-
- Bell, S. D., C. H. Botting, B. N. Wardleworth, S. P. Jackson, and M. F. White. 2002. The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science 296148-151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

