Growth phase- and cell division-dependent activation and inactivation of the {sigma}32 regulon in Escherichia coli
- PMID: 19114495
- PMCID: PMC2648200
- DOI: 10.1128/JB.01536-08
Growth phase- and cell division-dependent activation and inactivation of the {sigma}32 regulon in Escherichia coli
Abstract
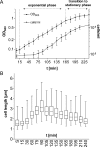
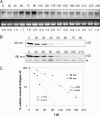
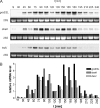
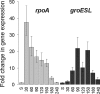
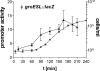
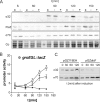
Alternative sigma factors allow bacteria to reprogram global transcription rapidly and to adapt to changes in the environment. Here we report on growth- and cell division-dependent sigma(32) regulon activity in Escherichia coli in batch culture. By analyzing sigma(32) expression in growing cells, an increase in sigma(32) protein levels is observed during the first round of cell division after exit from stationary phase. Increased sigma(32) protein levels result from transcriptional activation of the rpoH gene. After the first round of bulk cell division, rpoH transcript levels and sigma(32) protein levels decrease again. The late-logarithmic phase and the transition to stationary phase are accompanied by a second increase in sigma(32) levels and enhanced stability of sigma(32) protein but not by enhanced transcription of rpoH. Throughout growth, sigma(32) target genes show expression patterns consistent with oscillating sigma(32) protein levels. However, during the transition to early-stationary phase, despite high sigma(32) protein levels, the transcription of sigma(32) target genes is downregulated, suggesting functional inactivation of sigma(32). It is deduced from these data that there may be a link between sigma(32) regulon activity and cell division events. Further support for this hypothesis is provided by the observation that in cells in which FtsZ is depleted, sigma(32) regulon activation is suppressed.
Figures

References
-
- Avedissian, M., and S. Lopes Gomes. 1996. Expression of the groESL operon is cell-cycle controlled in Caulobacter crescentus. Mol. Microbiol. 1979-89. - PubMed
-
- Bi, E. F., and J. Lutkenhaus. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354161-164. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

