Efficacy of a vaccine based on protective antigen and killed spores against experimental inhalational anthrax
- PMID: 19114543
- PMCID: PMC2643630
- DOI: 10.1128/IAI.01217-08
Efficacy of a vaccine based on protective antigen and killed spores against experimental inhalational anthrax
Abstract
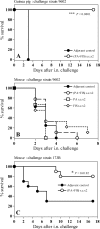
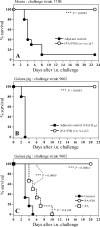
Protective antigen (PA)-based anthrax vaccines acting on toxins are less effective than live attenuated vaccines, suggesting that additional antigens may contribute to protective immunity. Several reports indicate that capsule or spore-associated antigens may enhance the protection afforded by PA. Addition of formaldehyde-inactivated spores (FIS) to PA (PA-FIS) elicits total protection against cutaneous anthrax. Nevertheless, vaccines that are effective against cutaneous anthrax may not be so against inhalational anthrax. The aim of this work was to optimize immunization with PA-FIS and to assess vaccine efficacy against inhalational anthrax. We assessed the immune response to recombinant anthrax PA from Bacillus anthracis (rPA)-FIS administered by various immunization protocols and the protection provided to mice and guinea pigs infected through the respiratory route with spores of a virulent strain of B. anthracis. Combined subcutaneous plus intranasal immunization of mice yielded a mucosal immunoglobulin G response to rPA that was more than 20 times higher than that in lung mucosal secretions after subcutaneous vaccination. The titers of toxin-neutralizing antibody and antispore antibody were also significantly higher: nine and eight times higher, respectively. The optimized immunization elicited total protection of mice intranasally infected with the virulent B. anthracis strain 17JB. Guinea pigs were fully protected, both against an intranasal challenge with 100 50% lethal doses (LD(50)) and against an aerosol with 75 LD(50) of spores of the highly virulent strain 9602. Conversely, immunization with PA alone did not elicit protection. These results demonstrate that the association of PA and spores is very much more effective than PA alone against experimental inhalational anthrax.
Figures



Similar articles
-
Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge.Infect Immun. 2007 Aug;75(8):4020-9. doi: 10.1128/IAI.00070-07. Epub 2007 May 14. Infect Immun. 2007. PMID: 17502384 Free PMC article.
-
Anthrax spores make an essential contribution to vaccine efficacy.Infect Immun. 2002 Feb;70(2):661-4. doi: 10.1128/IAI.70.2.661-664.2002. Infect Immun. 2002. PMID: 11796596 Free PMC article.
-
The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores.Microbiology (Reading). 2001 Jun;147(Pt 6):1677-1685. doi: 10.1099/00221287-147-6-1677. Microbiology (Reading). 2001. PMID: 11390699
-
Progress and novel strategies in vaccine development and treatment of anthrax.Immunol Rev. 2011 Jan;239(1):221-36. doi: 10.1111/j.1600-065X.2010.00969.x. Immunol Rev. 2011. PMID: 21198675 Review.
-
Anthrax vaccination strategies.Mol Aspects Med. 2009 Dec;30(6):490-502. doi: 10.1016/j.mam.2009.08.006. Epub 2009 Sep 1. Mol Aspects Med. 2009. PMID: 19729034 Free PMC article. Review.
Cited by
-
Noninvasive imaging technologies reveal edema toxin as a key virulence factor in anthrax.Am J Pathol. 2011 Jun;178(6):2523-35. doi: 10.1016/j.ajpath.2011.02.027. Am J Pathol. 2011. PMID: 21641378 Free PMC article.
-
Immunogenicity of Non-Living Anthrax Vaccine Candidates in Cattle and Protective Efficacy of Immune Sera in A/J Mouse Model Compared to the Sterne Live Spore Vaccine.Pathogens. 2020 Jul 10;9(7):557. doi: 10.3390/pathogens9070557. Pathogens. 2020. PMID: 32664259 Free PMC article.
-
Immunization of mice with formalin-inactivated spores from avirulent Bacillus cereus strains provides significant protection from challenge with Bacillus anthracis Ames.Clin Vaccine Immunol. 2013 Jan;20(1):56-65. doi: 10.1128/CVI.00550-12. Epub 2012 Oct 31. Clin Vaccine Immunol. 2013. PMID: 23114705 Free PMC article.
-
Immunogenicity and Protective Efficacy of a Non-Living Anthrax Vaccine versus a Live Spore Vaccine with Simultaneous Penicillin-G Treatment in Cattle.Vaccines (Basel). 2020 Oct 9;8(4):595. doi: 10.3390/vaccines8040595. Vaccines (Basel). 2020. PMID: 33050254 Free PMC article.
-
Progress toward the Development of a NEAT Protein Vaccine for Anthrax Disease.Infect Immun. 2016 Nov 18;84(12):3408-3422. doi: 10.1128/IAI.00755-16. Print 2016 Dec. Infect Immun. 2016. PMID: 27647868 Free PMC article.
References
-
- Adone, R., P. Pasquali, G. La Rosa, C. Marianelli, M. Muscillo, A. Fasanella, M. Francia, and F. Ciuchini. 2002. Sequence analysis of the genes encoding for the major virulence factors of Bacillus anthracis vaccine strain “Carbosap.” J. Appl. Microbiol. 93117-121. - PubMed
-
- Banks, D. J., M. Barnajian, F. J. Maldonado-Arocho, A. M. Sanchez, and K. A. Bradley. 2005. Anthrax toxin receptor 2 mediates Bacillus anthracis killing of macrophages following spore challenge. Cell. Microbiol. 71173-1185. - PubMed
-
- Berthier, M., J. L. Fauchere, J. Perrin, B. Grignon, and D. Oriot. 1996. Fulminant meningitis due to Bacillus anthracis in 11-year-old girl during Ramadan. Lancet 347828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

