Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum
- PMID: 19114544
- PMCID: PMC2643632
- DOI: 10.1128/IAI.01280-08
Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum
Abstract
The capacity of Neisseria gonorrhoeae to cause disseminated gonococcal infection requires that such strains resist the bactericidal action of normal human serum. The bactericidal action of normal human serum against N. gonorrhoeae is mediated by the classical complement pathway through an antibody-dependent mechanism. The mechanism(s) by which certain strains of gonococci resist normal human serum is not fully understood, but alterations in lipooligosaccharide structure can affect such resistance. During an investigation of the biological significance of phosphoethanolamine extensions from lipooligosaccharide, we found that phosphoethanolamine substitutions from the heptose II group of the lipooligosaccharide beta-chain did not impact levels of gonococcal (strain FA19) resistance to normal human serum or polymyxin B. However, loss of phosphoethanolamine substitution from the lipid A component of lipooligosaccharide, due to insertional inactivation of lptA, resulted in increased gonococcal susceptibility to polymyxin B, as reported previously for Neisseria meningitidis. In contrast to previous reports with N. meningitidis, loss of phosphoethanolamine attached to lipid A rendered strain FA19 susceptible to complement killing. Serum killing of the lptA mutant occurred through the classical complement pathway. Both serum and polymyxin B resistance as well as phosphoethanolamine decoration of lipid A were restored in the lptA-null mutant by complementation with wild-type lptA. Our results support a role for lipid A phosphoethanolamine substitutions in resistance of this strict human pathogen to innate host defenses.
Figures

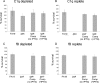

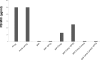
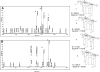
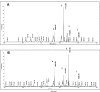
References
-
- Caroff, M., A. Tacken, and L. Szabo. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175273-282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

