Porphyromonas gingivalis RgpA-Kgp proteinase-adhesin complexes penetrate gingival tissue and induce proinflammatory cytokines or apoptosis in a concentration-dependent manner
- PMID: 19114547
- PMCID: PMC2643621
- DOI: 10.1128/IAI.01038-08
Porphyromonas gingivalis RgpA-Kgp proteinase-adhesin complexes penetrate gingival tissue and induce proinflammatory cytokines or apoptosis in a concentration-dependent manner
Abstract
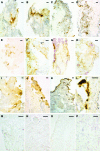
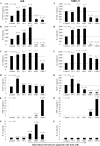
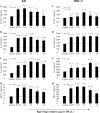
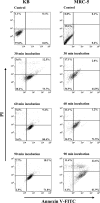
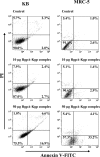
The RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis were observed, using immunostaining, in human gingival tissue associated with periodontitis but not in healthy tissue. The staining pattern suggested a concentration gradient from the subgingival plaque into the subjacent gingival connective tissue. Intense immunostaining was observed in areas displaying gross disturbance of tissue architecture. P. gingivalis cells and the RgpA-Kgp complexes at low concentrations were shown to stimulate secretory intercellular adhesion molecule 1, interleukin-8 (IL-8), IL-6, and macrophage chemoattractant protein secretion from cultured human epithelial (KB) and fibroblast (MRC-5) cells. However, at high concentrations a reduction in the level of these mediators was observed. In contrast, macrophage inflammatory protein 1alpha and IL-1alpha were stimulated only at high P. gingivalis cell concentrations. P. gingivalis cells and the RgpA-Kgp complexes were shown to induce apoptosis in KB and MRC-5 cells in a time- and dose-dependent manner. These data suggest that the RgpA-Kgp complexes penetrate the gingival connective tissue; at low concentrations distal from the plaque the complexes stimulate the secretion of proinflammatory mediators, while at high concentrations proximal to the plaque they induce apoptosis and attenuate the secretion of proinflammatory mediators.
Figures



References
-
- Ansai, T., E. Yamamoto, S. Awano, W. Yu, A. J. Turner, and T. Takehara. 2002. Effects of periodontopathic bacteria on the expression of endothelin-1 in gingival epithelial cells in adult periodontitis. Clin. Sci. (London) 103327S-331S. - PubMed
-
- Assuma, R., T. Oates, D. Cochran, S. Amar, and D. T. Graves. 1998. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 160403-409. - PubMed
-
- Aubry, J. P., A. Blaecke, S. Lecoanet-Henchoz, P. Jeannin, N. Herbault, G. Caron, V. Moine, and J. Y. Bonnefoy. 1999. Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry 37197-204. - PubMed
-
- Banbula, A., M. Bugno, A. Kuster, P. C. Heinrich, J. Travis, and J. Potempa. 1999. Rapid and efficient inactivation of IL-6 gingipains, lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Biochem. Biophys. Res. Commun. 261598-602. - PubMed
-
- Bascones, A., J. Gamonal, M. Gomez, A. Silva, and M. A. Gonzalez. 2004. New knowledge of the pathogenesis of periodontal disease. Quintessence Int. 35706-716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

