Requirement of RNA binding of mammalian eukaryotic translation initiation factor 4GI (eIF4GI) for efficient interaction of eIF4E with the mRNA cap
- PMID: 19114555
- PMCID: PMC2648241
- DOI: 10.1128/MCB.01187-08
Requirement of RNA binding of mammalian eukaryotic translation initiation factor 4GI (eIF4GI) for efficient interaction of eIF4E with the mRNA cap
Abstract
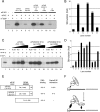
Eukaryotic mRNAs possess a 5'-terminal cap structure (cap), m(7)GpppN, which facilitates ribosome binding. The cap is bound by eukaryotic translation initiation factor 4F (eIF4F), which is composed of eIF4E, eIF4G, and eIF4A. eIF4E is the cap-binding subunit, eIF4A is an RNA helicase, and eIF4G is a scaffolding protein that bridges between the mRNA and ribosome. eIF4G contains an RNA-binding domain, which was suggested to stimulate eIF4E interaction with the cap in mammals. In Saccharomyces cerevisiae, however, such an effect was not observed. Here, we used recombinant proteins to reconstitute the cap binding of the mammalian eIF4E-eIF4GI complex to investigate the importance of the RNA-binding region of eIF4GI for cap interaction with eIF4E. We demonstrate that chemical cross-linking of eIF4E to the cap structure is dramatically enhanced by eIF4GI fragments possessing RNA-binding activity. Furthermore, the fusion of RNA recognition motif 1 (RRM1) of the La autoantigen to the N terminus of eIF4GI confers enhanced association between the cap structure and eIF4E. These results demonstrate that eIF4GI serves to anchor eIF4E to the mRNA and enhance its interaction with the cap structure.
Figures

Similar articles
-
Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of eIF4GI constitute the core domain for cap-dependent translation, and the C-terminal one-third functions as a modulatory region.Mol Cell Biol. 2000 Jan;20(2):468-77. doi: 10.1128/MCB.20.2.468-477.2000. Mol Cell Biol. 2000. PMID: 10611225 Free PMC article.
-
Inhibition of Mitogen-activated Protein Kinase (MAPK)-interacting Kinase (MNK) Preferentially Affects Translation of mRNAs Containing Both a 5'-Terminal Cap and Hairpin.J Biol Chem. 2016 Feb 12;291(7):3455-67. doi: 10.1074/jbc.M115.694190. Epub 2015 Dec 14. J Biol Chem. 2016. PMID: 26668315 Free PMC article.
-
Mechanistic differences in eukaryotic initiation factor requirements for eIF4GI-driven cap-independent translation of structured mRNAs.J Biol Chem. 2024 Nov;300(11):107866. doi: 10.1016/j.jbc.2024.107866. Epub 2024 Oct 9. J Biol Chem. 2024. PMID: 39384039 Free PMC article.
-
The translation of capped mRNAs has an absolute requirement for the central domain of eIF4G but not for the cap-binding initiation factor eIF4E.Cold Spring Harb Symp Quant Biol. 2001;66:377-87. doi: 10.1101/sqb.2001.66.377. Cold Spring Harb Symp Quant Biol. 2001. PMID: 12762040 Review. No abstract available.
-
Manipulation of the host translation initiation complex eIF4F by DNA viruses.Biochem Soc Trans. 2010 Dec;38(6):1511-6. doi: 10.1042/BST0381511. Biochem Soc Trans. 2010. PMID: 21118117 Review.
Cited by
-
Milk--A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation.Int J Mol Sci. 2015 Jul 27;16(8):17048-87. doi: 10.3390/ijms160817048. Int J Mol Sci. 2015. PMID: 26225961 Free PMC article. Review.
-
The poly(A)-binding protein partner Paip2a controls translation during late spermiogenesis in mice.J Clin Invest. 2010 Sep;120(9):3389-400. doi: 10.1172/JCI43350. Epub 2010 Aug 25. J Clin Invest. 2010. PMID: 20739757 Free PMC article.
-
Engineering a human-based translational activator for targeted protein expression restoration.bioRxiv [Preprint]. 2025 Jul 9:2025.07.09.663984. doi: 10.1101/2025.07.09.663984. bioRxiv. 2025. PMID: 40672171 Free PMC article. Preprint.
-
Regulation of neuronal mRNA translation by CaM-kinase I phosphorylation of eIF4GII.J Neurosci. 2012 Apr 18;32(16):5620-30. doi: 10.1523/JNEUROSCI.0030-12.2012. J Neurosci. 2012. PMID: 22514323 Free PMC article.
-
The mechanism of eukaryotic translation initiation: new insights and challenges.Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a011544. doi: 10.1101/cshperspect.a011544. Cold Spring Harb Perspect Biol. 2012. PMID: 22815232 Free PMC article. Review.
References
-
- Altmann, M., I. Edery, H. Trachsel, and N. Sonenberg. 1988. Site-directed mutagenesis of the tryptophan residues in yeast eukaryotic initiation factor 4E. Effects on cap binding activity. J. Biol. Chem. 26317229-17232. - PubMed
-
- Carberry, S. E., R. E. Rhoads, and D. J. Goss. 1989. A spectroscopic study of the binding of m7GTP and m7GpppG to human protein synthesis initiation factor 4E. Biochemistry 288078-8083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
