Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site
- PMID: 19114638
- PMCID: PMC2606938
- DOI: 10.1085/jgp.200810154
Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site
Abstract
Paracellular ion transport in epithelia is mediated by pores formed by members of the claudin family. The degree of selectivity and the molecular mechanism of ion permeation through claudin pores are poorly understood. By expressing a high-conductance claudin isoform, claudin-2, in high-resistance Madin-Darby canine kidney cells under the control of an inducible promoter, we were able to quantitate claudin pore permeability. Claudin-2 pores were found to be narrow, fluid filled, and cation selective. Charge selectivity was mediated by the electrostatic interaction of partially dehydrated permeating cations with a negatively charged site within the pore that is formed by the side chain carboxyl group of aspartate-65. Thus, paracellular pores use intrapore electrostatic binding sites to achieve a high conductance with a high degree of charge selectivity.
Figures




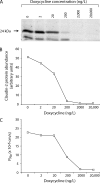



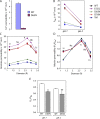
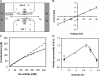

References
-
- Alexandre, M.D., Q. Lu, and Y.H. Chen. 2005. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 118:2683–2693. - PubMed
-
- Amasheh, S., N. Meiri, A.H. Gitter, T. Schoneberg, J. Mankertz, J.D. Schulzke, and M. Fromm. 2002. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115:4969–4976. - PubMed
-
- Angelow, S., R. El-Husseini, S.A. Kanzawa, and A.S. Yu. 2007. a. Renal localization and function of the tight junction protein, claudin-19. Am. J. Physiol. Renal Physiol. 293:F166–F177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

