Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment
- PMID: 19114666
- PMCID: PMC2626680
- DOI: 10.1084/jem.20080129
Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment
Abstract
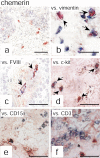
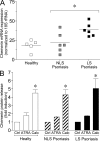
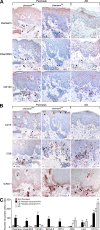
Psoriasis is a type I interferon-driven T cell-mediated disease characterized by the recruitment of plasmacytoid dendritic cells (pDC) into the skin. The molecules involved in pDC accumulation in psoriasis lesions are unknown. Chemerin is the only inflammatory chemotactic factor that is directly active on human blood pDC in vitro. The aim of this study was to evaluate the role of the chemerin/ChemR23 axis in the recruitment of pDC in psoriasis skin. Prepsoriatic skin adjacent to active lesions and early lesions were characterized by a strong expression of chemerin in the dermis and by the presence of CD15(+) neutrophils and CD123(+)/BDCA-2(+)/ChemR23(+) pDC. Conversely, skin from chronic plaques showed low chemerin expression, segregation of neutrophils to epidermal microabscesses, and few pDC in the dermis. Chemerin expression was localized mainly in fibroblasts, mast cells, and endothelial cells. Fibroblasts cultured from skin of psoriatic lesions expressed higher levels of chemerin messenger RNA and protein than fibroblasts from uninvolved psoriatic skin or healthy donors and promoted pDC migration in vitro in a chemerin-dependent manner. Therefore, chemerin expression specifically marks the early phases of evolving skin psoriatic lesions and is temporally strictly associated with pDC. These results support a role for the chemerin/ChemR23 axis in the early phases of psoriasis development.
Figures




References
-
- Colonna, M., G. Trinchieri, and Y.J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226. - PubMed
-
- Liu, Y.J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275–306. - PubMed
-
- Colonna, M., and M. Cella. 2007. Crosspresentation: plasmacytoid dendritic cells are in the business. Immunity. 27:419–421. - PubMed
-
- Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

