Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma
- PMID: 19114704
- PMCID: PMC2645088
- DOI: 10.1200/JCO.2008.16.3055
Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma
Abstract
Purpose: To evaluate single-agent activity of bevacizumab in patients with recurrent glioblastoma.
Patients and methods: Patients with recurrent glioblastoma were treated with bevacizumab 10 mg/kg every 2 weeks. After tumor progression, patients were immediately treated with bevacizumab in combination with irinotecan 340 mg/m(2) or 125 mg/m(2) every 2 weeks, depending on use of enzyme-inducing antiepileptic drugs. Complete patient evaluations were repeated every 4 weeks.
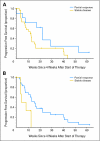
Results: Forty-eight heavily pretreated patients were accrued to this study. Thromboembolic events (12.5%), hypertension (12.5%), hypophosphatemia (6%), and thrombocytopenia (6%) were the most common drug-associated adverse events. Six patients (12.5%) were removed from study for drug-associated toxicity (five thromboembolic events, one bowel perforation). Thirty-four patients (71%) and 17 patients (35%) achieved radiographic response based on Levin and Macdonald criteria, respectively. Median progression-free survival (PFS) was 16 weeks (95% CI, 12 to 26 weeks). The 6-month PFS was 29% (95% CI, 18% to 48%). The 6-month overall survival was 57% (95% CI, 44% to 75%). Median overall survival was 31 weeks (95% CI, 21 to 54 weeks). Early magnetic resonance imaging response (first 96 hours and 4 weeks) was predictive of long-term PFS, with the Levin criteria being more predictive than Macdonald criteria. Of 19 patients treated with bevacizumab plus irinotecan at progression, there were no objective radiographic responses. Eighteen patients (95%) experienced disease progression by the second cycle, and the median PFS was 30 days.
Conclusion: We conclude that single-agent bevacizumab has significant biologic and antiglioma activity in patients with recurrent glioblastoma.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Takahashi JA, Fukumoto M, Igarashi K, et al. Correlation of basic fibroblast growth factor expression levels with the degree of malignancy and vascularity in human gliomas. J Neurosurg. 1992;76:792–798. - PubMed
-
- Maxwell M, Naber SP, Wolfe HJ, et al. Expression of angiogenic growth factor genes in primary human astrocytomas may contribute to their growth and progression. Cancer Res. 1991;51:1345–1351. - PubMed
-
- Stefanik DF, Fellows WK, Rizkalla LR, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001;55:91–100. - PubMed
-
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

