Akt regulates drug-induced cell death through Bcl-w downregulation
- PMID: 19114998
- PMCID: PMC2603590
- DOI: 10.1371/journal.pone.0004070
Akt regulates drug-induced cell death through Bcl-w downregulation
Retraction in
-
Retraction: Akt Regulates Drug-Induced Cell Death through Bcl-w Downregulation.PLoS One. 2022 Apr 20;17(4):e0267621. doi: 10.1371/journal.pone.0267621. eCollection 2022. PLoS One. 2022. PMID: 35442994 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern: Akt Regulates Drug-Induced Cell Death through Bcl-w Downregulation.PLoS One. 2019 Mar 19;14(3):e0213701. doi: 10.1371/journal.pone.0213701. eCollection 2019. PLoS One. 2019. PMID: 30889206 Free PMC article. No abstract available.
Abstract
Akt is a serine threonine kinase with a major role in transducing survival signals and regulating proteins involved in apoptosis. To find new interactors of Akt involved in cell survival, we performed a two-hybrid screening in yeast using human full-length Akt c-DNA as bait and a murine c-DNA library as prey. Among the 80 clones obtained, two were identified as Bcl-w. Bcl-w is a member of the Bcl-2 family that is essential for the regulation of cellular survival, and that is up-regulated in different human tumors, such as gastric and colorectal carcinomas. Direct interaction of Bcl-w with Akt was confirmed by immunoprecipitation assays. Subsequently, we addressed the function of this interaction: by interfering with the activity or amount of Akt, we have demonstrated that Akt modulates the amount of Bcl-w protein. We have found that inhibition of Akt activity may promote apoptosis through the downregulation of Bcl-w protein and the consequential reduction in interaction of Bcl-w with pro-apoptotic members of the Bcl-2 family. Our data provide evidence that Bcl-w is a new member of the Akt pathway and that Akt may induce anti-apoptotic signals at least in part through the regulation of the amount and activity of Bcl-w.
Conflict of interest statement
Figures




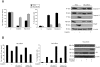

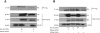
References
-
- Bellacosa A, Franke TF, Gonzalez-Portal ME, Datta K, Taguchi T, et al. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 1993;8:745–754. - PubMed
-
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. - PubMed
-
- Yoeli-Lerner M, Toker A. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle. 2006;5:603–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

