Cell surface heparan sulfate released by heparanase promotes melanoma cell migration and angiogenesis
- PMID: 19115257
- PMCID: PMC2736788
- DOI: 10.1002/jcb.22005
Cell surface heparan sulfate released by heparanase promotes melanoma cell migration and angiogenesis
Abstract
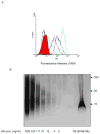
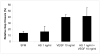
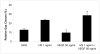
Heparan sulfate (HS) proteoglycans are essential components of the cell-surface and extracellular matrix (ECM) which provide structural integrity and act as storage depots for growth factors and chemokines, through their HS side chains. Heparanase (HPSE) is the only mammalian endoglycosidase known that cleaves HS, thus contributing to matrix degradation and cell invasion. The enzyme acts as an endo-beta-D-glucuronidase resulting in HS fragments of discrete molecular weight size. Cell-surface HS is known to inhibit or stimulate tumorigenesis depending upon size and composition. We hypothesized that HPSE contributes to melanoma metastasis by generating bioactive HS from the cell-surface to facilitate biological activities of tumor cells as well as tumor microenvironment. We removed cell-surface HS from melanoma (B16B15b) by HPSE treatment and resulting fragments were isolated. Purified cell-surface HS stimulated in vitro B16B15b cell migration but not proliferation, and importantly, enhanced in vivo angiogenesis. Furthermore, melanoma cell-surface HS did not affect in vitro endothelioma cell (b.End3) migration. Our results provide direct evidence that, in addition to remodeling ECM and releasing growth factors and chemokines, HPSE contributes to aggressive phenotype of melanoma by releasing bioactive cell-surface HS fragments which can stimulate melanoma cell migration in vitro and angiogenesis in vivo.
Figures



Similar articles
-
Heparanase degrades syndecan-1 and perlecan heparan sulfate: functional implications for tumor cell invasion.J Biol Chem. 2004 Feb 27;279(9):8047-55. doi: 10.1074/jbc.M304872200. Epub 2003 Nov 20. J Biol Chem. 2004. PMID: 14630925
-
A functional heparan sulfate mimetic implicates both heparanase and heparan sulfate in tumor angiogenesis and invasion in a mouse model of multistage cancer.Oncogene. 2005 Jun 9;24(25):4037-51. doi: 10.1038/sj.onc.1208602. Oncogene. 2005. PMID: 15806157
-
Heparanase and a synthetic peptide of heparan sulfate-interacting protein recognize common sites on cell surface and extracellular matrix heparan sulfate.J Biol Chem. 1997 Jun 20;272(25):15891-7. doi: 10.1074/jbc.272.25.15891. J Biol Chem. 1997. PMID: 9188488
-
Chemical toolbox to interrogate Heparanase-1 activity.Curr Opin Chem Biol. 2024 Jun;80:102452. doi: 10.1016/j.cbpa.2024.102452. Epub 2024 Mar 30. Curr Opin Chem Biol. 2024. PMID: 38555836 Review.
-
Heparanase: Historical Aspects and Future Perspectives.Adv Exp Med Biol. 2020;1221:71-96. doi: 10.1007/978-3-030-34521-1_3. Adv Exp Med Biol. 2020. PMID: 32274707 Review.
Cited by
-
Heparanase released from mesenchymal stem cells activates integrin beta1/HIF-2alpha/Flk-1 signaling and promotes endothelial cell migration and angiogenesis.Stem Cells. 2015 Jun;33(6):1850-1862. doi: 10.1002/stem.1995. Stem Cells. 2015. PMID: 25754303 Free PMC article.
-
Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan.Front Oncol. 2020 Jan 17;9:1482. doi: 10.3389/fonc.2019.01482. eCollection 2019. Front Oncol. 2020. PMID: 32010611 Free PMC article. Review.
-
The Roles of Angiogenesis in Malignant Melanoma: Trends in Basic Science Research over the Last 100 Years.ISRN Oncol. 2012;2012:546927. doi: 10.5402/2012/546927. Epub 2012 Jun 7. ISRN Oncol. 2012. PMID: 22720169 Free PMC article.
-
Role of the blood-brain barrier in the formation of brain metastases.Int J Mol Sci. 2013 Jan 11;14(1):1383-411. doi: 10.3390/ijms14011383. Int J Mol Sci. 2013. PMID: 23344048 Free PMC article. Review.
-
Tumor Microenvironment-Associated Extracellular Matrix Components Regulate NK Cell Function.Front Immunol. 2020 Jan 29;11:73. doi: 10.3389/fimmu.2020.00073. eCollection 2020. Front Immunol. 2020. PMID: 32063906 Free PMC article. Review.
References
-
- Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: Growth regulation and the prospect of new cancer therapies. J Biol Chem. 2005;96:897–905. - PubMed
-
- Elkin M, Ilan M, Ishai-Michaeli R, Friedmann Y, Pappo O, Pecker I, Vlodavsky I. Heparanase as mediator of angiogenesis: mode of action. FASEB J. 2001;15(9):1661–1663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

