VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice
- PMID: 19115316
- PMCID: PMC3756672
- DOI: 10.1002/hep.22711
VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice
Abstract
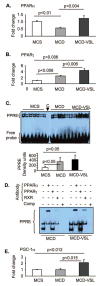
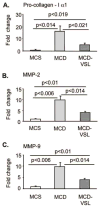
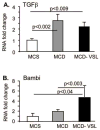
Nonalcoholic fatty liver disease (NAFLD) and its advanced stage, nonalcoholic steatohepatitis (NASH), are the most common causes of chronic liver disease in the United States. NASH features the metabolic syndrome, inflammation, and fibrosis. Probiotics exhibit immunoregulatory and anti-inflammatory activity. We tested the hypothesis that probiotic VSL#3 may ameliorate the methionine-choline-deficient (MCD) diet-induced mouse model of NASH. MCD diet resulted in NASH in C57BL/6 mice compared to methionine-choline-supplemented (MCS) diet feeding evidenced by liver steatosis, increased triglycerides, inflammatory cell accumulation, increased tumor necrosis factor alpha levels, and fibrosis. VSL#3 failed to prevent MCD-induced liver steatosis or inflammation. MCD diet, even in the presence of VSL#3, induced up-regulation of serum endotoxin and expression of the Toll-like receptor 4 signaling components, including CD14 and MD2, MyD88 adaptor, and nuclear factor kappaB activation. In contrast, VSL#3 treatment ameliorated MCD diet-induced liver fibrosis resulting in diminished accumulation of collagen and alpha-smooth muscle actin. We identified increased expression of liver peroxisome proliferator-activated receptors and decreased expression of procollagen and matrix metalloproteinases in mice fed MCD+VSL#3 compared to MCD diet alone. MCD diet triggered up-regulation of transforming growth factor beta (TGFbeta), a known profibrotic agent. In the presence of VSL#3, the MCD diet-induced expression of TGFbeta was maintained; however, the expression of Bambi, a TGFbeta pseudoreceptor with negative regulatory function, was increased. In summary, our data indicate that VSL#3 modulates liver fibrosis but does not protect from inflammation and steatosis in NASH. The mechanisms of VSL#3-mediated protection from MCD diet-induced liver fibrosis likely include modulation of collagen expression and impaired TGFbeta signaling.
Figures



References
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. - PubMed
-
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. - PubMed
-
- Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat? J Hepatol. 2006;44:253–261. - PubMed
-
- Farrell GC, Larter CZ. Nonalcholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. - PubMed
-
- Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2006;343:1467–1476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
