Sphingomonas paucimobilis bloodstream infections associated with contaminated intravenous fentanyl
- PMID: 19116043
- PMCID: PMC2660711
- DOI: 10.3201/eid1501.081054
Sphingomonas paucimobilis bloodstream infections associated with contaminated intravenous fentanyl
Abstract
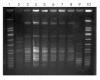
Nationally distributed medications from compounding pharmacies, which typically adhere to less stringent quality-control standards than pharmaceutical manufacturers, can lead to multistate outbreaks. We investigated a cluster of 6 patients in a Maryland hospital who had Sphingomonas paucimobilis bloodstream infections in November 2007. Of the 6 case-patients, 5 (83%) had received intravenous fentanyl within 48 hours before bacteremia developed. Cultures of unopened samples of fentanyl grew S. paucimobilis; the pulsed-field gel electrophoresis pattern was indistinguishable from that of the isolates of 5 case-patients. The contaminated fentanyl lot had been prepared at a compounding pharmacy and distributed to 4 states. Subsequently, in California, S. paucimobilis bacteremia was diagnosed for 2 patients who had received intravenous fentanyl from the same compounding pharmacy. These pharmacies should adopt more stringent quality-control measures, including prerelease product testing, when compounding and distributing large quantities of sterile preparations.
Figures

References
-
- US Food and Drug Administration. Guidance for FDA staff and industry compliance policy guides manual; section 460.200; pharmacy compounding; 2002. [cited 2008 Mar 28]. Available from http://www.fda.gov/OHRMS/DOCKETS/98fr/02D-0242_gdl0001.pdf
-
- Sunenshine RH, Tan ET, Terashita DM, Jensen BJ, Kacica MA, Sickbert-Bennett EE, et al. A multistate outbreak of Serratia marcescens bloodstream infection associated with contaminated intravenous magnesium sulfate from a compounding pharmacy. Clin Infect Dis. 2007;45:527–33. 10.1086/520664 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
