The subcellular distribution of an RNA quality control protein, the Ro autoantigen, is regulated by noncoding Y RNA binding
- PMID: 19116308
- PMCID: PMC2649258
- DOI: 10.1091/mbc.e08-11-1094
The subcellular distribution of an RNA quality control protein, the Ro autoantigen, is regulated by noncoding Y RNA binding
Abstract
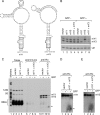
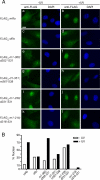
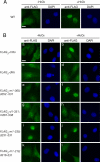
The Ro autoantigen is a ring-shaped RNA-binding protein that binds misfolded RNAs in nuclei and is proposed to function in quality control. In the cytoplasm, Ro binds noncoding RNAs, called Y RNAs, that inhibit access of Ro to other RNAs. Ro also assists survival of mammalian cells and at least one bacterium after UV irradiation. In mammals, Ro undergoes dramatic localization changes after UV irradiation, changing from mostly cytoplasmic to predominantly nuclear. Here, we report that a second role of Y RNAs is to regulate the subcellular distribution of Ro. A mutant Ro protein that does not bind Y RNAs accumulates in nuclei. Ro also localizes to nuclei when Y RNAs are depleted. By assaying chimeric proteins in which portions of mouse Ro were replaced with bacterial Ro sequences, we show that nuclear accumulation of Ro after irradiation requires sequences that overlap the Y RNA binding site. Ro also accumulates in nuclei after oxidative stress, and similar sequences are required. Together, these data reveal that Ro contains a signal for nuclear accumulation that is masked by a bound Y RNA and suggest that Y RNA binding may be modulated during cell stress.
Figures




References
-
- Chen X., Smith J. D., Shi H., Yang D. D., Flavell R. A., Wolin S. L. The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr. Biol. 2003;13:2206–2211. - PubMed
-
- Chen X., Wolin S. L. The Ro 60 kDa autoantigen: insights into cellular function and role in autoimmunity. J. Mol. Med. 2004;82:232–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

