The conserved RIC-3 coiled-coil domain mediates receptor-specific interactions with nicotinic acetylcholine receptors
- PMID: 19116311
- PMCID: PMC2649256
- DOI: 10.1091/mbc.e08-08-0851
The conserved RIC-3 coiled-coil domain mediates receptor-specific interactions with nicotinic acetylcholine receptors
Abstract
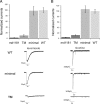
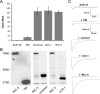
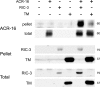
RIC-3 belongs to a conserved family of proteins influencing nicotinic acetylcholine receptor (nAChR) maturation. RIC-3 proteins are integral membrane proteins residing in the endoplasmic reticulum (ER), and containing a C-terminal coiled-coil domain (CC-I). Conservation of CC-I in all RIC-3 family members indicates its importance; however, previous studies could not show its function. To examine the role of CC-I, we studied effects of its deletion on Caenorhabditis elegans nAChRs in vivo. Presence of CC-I promoted maturation of particular nAChRs expressed in body-wall muscle, whereas it was not required for other nAChR subtypes expressed in neurons or pharyngeal muscles. This effect is receptor-specific, because it could be reproduced after heterologous expression. Consistently, coimmunoprecipitation analysis showed that CC-I enhances the interaction of RIC-3 with a nAChR that requires CC-I in vivo; thus CC-I appears to enhance affinity of RIC-3 to specific nAChRs. However, we found that this function of CC-I is redundant with functions of sequences downstream to CC-I, potentially a second coiled-coil. Alternative splicing in both vertebrates and invertebrates generates RIC-3 transcripts that lack the entire C-terminus, or only CC-I. Thus, our results suggest that RIC-3 alternative splicing enables subtype specific regulation of nAChR maturation.
Figures

References
-
- Ballivet M., Alliod C., Bertrand S., Bertrand D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J. Mol. Biol. 1996;258:261–269. - PubMed
-
- Castillo M., Mulet J., Gutierrez L. M., Ortiz J. A., Castelan F., Gerber S., Sala S., Sala F., Criado M. Dual role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280:27062–27068. - PubMed
-
- Cheng A., Bollan K. A., Greenwood S. M., Irving A. J., Connolly C. N. Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J. Biol. Chem. 2007;282:26158–26166. - PubMed
-
- Cheng A., McDonald N. A., Connolly C. N. Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J. Biol. Chem. 2005;280:22502–22507. - PubMed
-
- Cohen Ben-Ami H., Yassin L., Farah H., Michaeli A., Eshel M., Treinin M. RIC-3 affects properties and quantity of nicotinic acetylcholine receptors via a mechanism that does not require the coiled-coil domains. J. Biol. Chem. 2005;280:28053–28060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

