Genome-wide analysis of AP-3-dependent protein transport in yeast
- PMID: 19116312
- PMCID: PMC2649279
- DOI: 10.1091/mbc.e08-08-0819
Genome-wide analysis of AP-3-dependent protein transport in yeast
Abstract
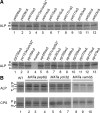
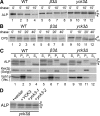
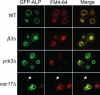
The evolutionarily conserved adaptor protein-3 (AP-3) complex mediates cargo-selective transport to lysosomes and lysosome-related organelles. To identify proteins that function in AP-3-mediated transport, we performed a genome-wide screen in Saccharomyces cerevisiae for defects in the vacuolar maturation of alkaline phosphatase (ALP), a cargo of the AP-3 pathway. Forty-nine gene deletion strains were identified that accumulated precursor ALP, many with established defects in vacuolar protein transport. Maturation of a vacuolar membrane protein delivered via a separate, clathrin-dependent pathway, was affected in all strains except those with deletions of YCK3, encoding a vacuolar type I casein kinase; SVP26, encoding an endoplasmic reticulum (ER) export receptor for ALP; and AP-3 subunit genes. Subcellular fractionation and fluorescence microscopy revealed ALP transport defects in yck3Delta cells. Characterization of svp26Delta cells revealed a role for Svp26p in ER export of only a subset of type II membrane proteins. Finally, ALP maturation kinetics in vac8Delta and vac17Delta cells suggests that vacuole inheritance is important for rapid generation of proteolytically active vacuolar compartments in daughter cells. We propose that the cargo-selective nature of the AP-3 pathway in yeast is achieved by AP-3 and Yck3p functioning in concert with machinery shared by other vacuolar transport pathways.
Figures




References
-
- Avaro S., Belgareh-Touze N., Sibella-Arguelles C., Volland C., Haguenauer- Tsapis R. Mutants defective in secretory/vacuolar pathways in the EUROFAN collection of yeast disruptants. Yeast. 2002;19:351–371. - PubMed
-
- Berger A. C., Salazar G., Styers M. L., Newell-Litwa K. A., Werner E., Maue R. A., Corbett A. H., Faundez V. The subcellular localization of the Niemann-Pick Type C proteins depends on the adaptor complex AP-3. J. Cell Sci. 2007;120:3640–3652. - PubMed
-
- Bonifacino J. S. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

