Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin
- PMID: 19116368
- PMCID: PMC2631323
- DOI: 10.2353/ajpath.2009.080599
Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin
Abstract
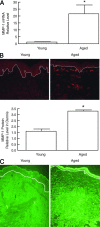
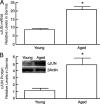
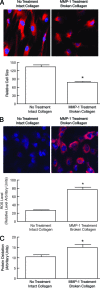
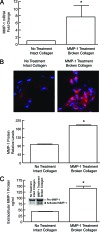
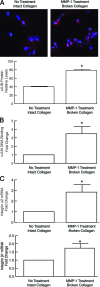
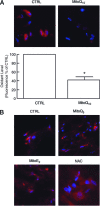
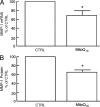
Aged human skin is fragile because of fragmentation and loss of type I collagen fibrils, which confer strength and resiliency. We report here that dermal fibroblasts express increased levels of collagen-degrading matrix metalloproteinases-1 (MMP-1) in aged (>80 years old) compared with young (21 to 30 years old) human skin in vivo. Transcription factor AP-1 and alpha2beta1 integrin, which are key regulators of MMP-1 expression, are also elevated in fibroblasts in aged human skin in vivo. MMP-1 treatment of young skin in organ culture causes fragmentation of collagen fibrils and reduces fibroblast stretch, consistent with reduced mechanical tension, as observed in aged human skin. Limited fragmentation of three-dimensional collagen lattices with exogenous MMP-1 also reduces fibroblast stretch and mechanical tension. Furthermore, fibroblasts cultured in fragmented collagen lattices express elevated levels of MMP-1, AP-1, and alpha2beta1 integrin. Importantly, culture in fragmented collagen raises intracellular oxidant levels and treatment with antioxidant MitoQ(10) significantly reduces MMP-1 expression. These data identify positive feedback regulation that couples age-dependent MMP-1-catalyzed collagen fragmentation and oxidative stress. We propose that this self perpetuating cycle promotes human skin aging. These data extend the current understanding of the oxidative theory of aging beyond a cellular-centric view to include extracellular matrix and the critical role that connective tissue microenvironment plays in the biology of aging.
Figures
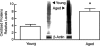



Similar articles
-
Expression of catalytically active matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin.Aging Cell. 2013 Aug;12(4):661-71. doi: 10.1111/acel.12089. Epub 2013 May 15. Aging Cell. 2013. PMID: 23601157 Free PMC article.
-
Age-associated reduction of cellular spreading/mechanical force up-regulates matrix metalloproteinase-1 expression and collagen fibril fragmentation via c-Jun/AP-1 in human dermal fibroblasts.Aging Cell. 2014 Dec;13(6):1028-37. doi: 10.1111/acel.12265. Epub 2014 Sep 9. Aging Cell. 2014. PMID: 25201474 Free PMC article.
-
Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo.Br J Dermatol. 2017 Nov;177(5):1337-1348. doi: 10.1111/bjd.15379. Epub 2017 Nov 1. Br J Dermatol. 2017. PMID: 28196296 Free PMC article.
-
Looking older: fibroblast collapse and therapeutic implications.Arch Dermatol. 2008 May;144(5):666-72. doi: 10.1001/archderm.144.5.666. Arch Dermatol. 2008. PMID: 18490597 Free PMC article. Review.
-
Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging.J Cell Commun Signal. 2018 Mar;12(1):35-43. doi: 10.1007/s12079-018-0459-1. Epub 2018 Feb 17. J Cell Commun Signal. 2018. PMID: 29455303 Free PMC article. Review.
Cited by
-
Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts.PLoS One. 2014 Dec 23;9(12):e115402. doi: 10.1371/journal.pone.0115402. eCollection 2014. PLoS One. 2014. PMID: 25536346 Free PMC article.
-
Age-related changes in dermal collagen physical properties in human skin.PLoS One. 2023 Dec 8;18(12):e0292791. doi: 10.1371/journal.pone.0292791. eCollection 2023. PLoS One. 2023. PMID: 38064445 Free PMC article.
-
Cellular Senescence and Inflammaging in the Skin Microenvironment.Int J Mol Sci. 2021 Apr 8;22(8):3849. doi: 10.3390/ijms22083849. Int J Mol Sci. 2021. PMID: 33917737 Free PMC article. Review.
-
The role of cellular senescence in skin aging and age-related skin pathologies.Front Physiol. 2023 Nov 22;14:1297637. doi: 10.3389/fphys.2023.1297637. eCollection 2023. Front Physiol. 2023. PMID: 38074322 Free PMC article. Review.
-
Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells.Antioxidants (Basel). 2021 May 5;10(5):726. doi: 10.3390/antiox10050726. Antioxidants (Basel). 2021. PMID: 34063059 Free PMC article. Review.
References
-
- Uitto J, Pulkkinen L, Chu M-L. Collagen. Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. New York: McGraw-Hill,; Dermatology in General Medicine. 2003:pp 165–179.
-
- Smith J, Davidson E, Sams W, Clark R. Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol. 1962;39:347–350. - PubMed
-
- Uitto J. Biology of dermal cells and extracellular matrix. Fitzpatrick T, Eisen A, Wolff K, Freedberg I, Austen K, editors. New York: McGraw-Hill,; Dermatology in General Medicine. 1993:pp 299–314.
-
- Warren R, Gartstein V, Kligman A, Montagna W, Allendorf R, Ridder G. Age, sunlight, and facial skin: a histologic and quantitative study. J Am Acad Dermatol. 1991;25:751–760. - PubMed
-
- West M. The cellular and molecular biology of skin aging. Arch Dermatol. 1994;130:87–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

