Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism
- PMID: 19116371
- PMCID: PMC3735925
- DOI: 10.1152/ajpendo.90949.2008
Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism
Abstract
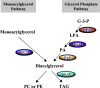
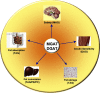
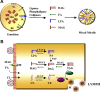
Monoacyglycerol acyltransferases (MGATs) and diacylglycerol acyltransferases (DGATs) catalyze two consecutive steps of enzyme reactions in the synthesis of triacylglycerols (TAGs). The metabolic complexity of TAG synthesis is reflected by the presence of multiple isoforms of MGAT and DGAT enzymes that differ in catalytic properties, subcellular localization, tissue distribution, and physiological functions. MGAT and DGAT enzymes play fundamental roles in the metabolism of monoacylglycerol (MAG), diacylglycerol (DAG), and triacylglycerol (TAG) that are involved in many aspects of physiological functions, such as intestinal fat absorption, lipoprotein assembly, adipose tissue formation, signal transduction, satiety, and lactation. The recent progress in the phenotypic characterization of mice deficient in MGAT and DGAT enzymes and the development of chemical inhibitors have revealed important roles of these enzymes in the regulation of energy homeostasis and insulin sensitivity. Consequently, selective inhibition of MGAT or DGAT enzymes by synthetic compounds may provide novel treatment for obesity and its related metabolic complications.
Figures

References
-
- Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab 14: 214–221, 2003 - PubMed
-
- Aguirre V, Uchida T, Yenush L, Davis R, White M. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000 - PubMed
-
- Angiolillo A, Amills M, Urrutia B, Domenech A, Sastre Y, Badaoui B, Jordana J. Identification of a single nucleotide polymorphism at intron 16 of the caprine acyl-coenzyme A: diacylglycerol acyltransferase 1 (DGAT1) gene. J Dairy Res 74: 47–51, 2007 - PubMed
-
- Avignon A, Yamada K, Zhou X, Spencer B, Cardona O, Saba-Siddique S, Galloway L, Standaert M, Farese R. Chronic activation of protein kinase C in soleus muscles and other tissues of insulin-resistant type II diabetic Goto-Kakizaki (GK), obese/aged, and obese/Zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes 45: 1396–1404, 1996 - PubMed
-
- Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem 49: 459–487, 1980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

