Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea
- PMID: 19116615
- PMCID: PMC3326399
- DOI: 10.1038/nrmicro2053
Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea
Abstract
Infectious diarrhoea is a significant contributor to morbidity and mortality worldwide. In bacterium-induced diarrhoea, rapid loss of fluids and electrolytes results from inhibition of the normal absorptive function of the intestine as well as the activation of secretory processes. Advances in the past 10 years in the fields of gastrointestinal physiology, innate immunity and enteric bacterial virulence mechanisms highlight the multifactorial nature of infectious diarrhoea. This review explores the various mechanisms that contribute to loss of fluids and electrolytes following bacterial infections, and attempts to link these events to specific virulence factors and toxins.
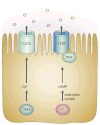
Figures



References
-
- Cheng AC, McDonald JR, Thielman NM. Infectious diarrhea in developed and developing countries. J Clin Gastroenterol. 2005;39:757–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

