Conformational Closure of the Catalytic Site of Human CD38 Induced by Calcium
- PMID: 19117080
- PMCID: PMC3732380
Conformational Closure of the Catalytic Site of Human CD38 Induced by Calcium
Abstract
First identified on the surface of lymphoids as a type II transmembrane protein, CD38 has now been established to have dual functions not only as a receptor but also as a multifunctional enzyme,catalyzing the synthesis of and hydrolysis of a general calcium messenger molecule, cyclic ADP-ribose(cADPR). The receptorial functions of CD38 include the induction of cell adhesion, differentiation,apoptosis, and cytokine production upon antibody ligation. Here we determined the crystal structure of calcium-loaded human CD38 at 1.45 A resolution which reveals that CD38 undergoes dramatic structural changes to an inhibited conformation in the presence of calcium. The structural changes are highly localized and occur in only two regions. The first region is part of the active site and consists of residues 121-141.In the presence of calcium, W125 moves 5 A into the active site and forms hydrophobic interactions with W189. The movement closes the active site pocket and reduces entry of substrates, resulting in inhibition of the enzymatic activity. The structural role of calcium in inducing these conformational changes is readily visualized in the crystal structure. The other region that undergoes calcium-induced changes is at the receptor region, where a highly ordered helix is unraveled to a random coil. The results suggest a novel conformational coupling mechanism, whereby protein interaction targeted at the receptor region can effectively regulate the enzymatic activity of CD38.
Figures

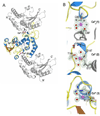
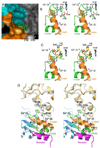

References
-
- Jackson DG, Bell JI. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144:2811–2815. - PubMed
-
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. - PubMed
-
- Aarhus R, Graeff RM, Dickey DM, Walseth TF, Lee HC. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem. 1995;270:30327–30333. - PubMed
-
- Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–1164. - PubMed
-
- Graeff R, Liu Q, Kriksunov IA, Hao Q, Lee HC. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem. 2006;281:28951–28957. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
