Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice
- PMID: 19117914
- PMCID: PMC2632856
- DOI: 10.1101/lm.1112209
Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice
Abstract
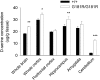
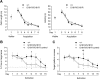
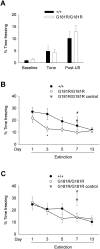
Activation of the N-methyl-D-aspartate receptor (NMDAR) glycine site has been shown to accelerate adaptive forms of learning that may benefit psychopathologies involving cognitive and perseverative disturbances. In this study, the effects of increasing the brain levels of the endogenous NMDAR glycine site agonist D-serine, through the genetic inactivation of its catabolic enzyme D-amino acid oxidase (DAO), were examined in behavioral tests of learning and memory. In the Morris water maze task (MWM), mice carrying the hypofunctional Dao1(G181R) mutation demonstrated normal acquisition of a single platform location but had substantially improved memory for a new target location in the subsequent reversal phase. Furthermore, Dao1(G181R) mutant animals exhibited an increased rate of extinction in the MWM that was similarly observed following pharmacological administration of D-serine (600 mg/kg) in wild-type C57BL/6J mice. In contextual and cued fear conditioning, no alterations were found in initial associative memory recall; however, extinction of the contextual fear memory was facilitated in mutant animals. Thus, an augmented level of D-serine resulting from reduced DAO activity promotes adaptive learning in response to changing conditions. The NMDAR glycine site and DAO may be promising therapeutic targets to improve cognitive flexibility and inhibitory learning in psychiatric disorders such as schizophrenia and anxiety syndromes.
Figures

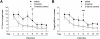
References
-
- Almond S.L., Fradley R.L., Armstrong E.J., Heavens R.B., Rutter A.R., Newman R.J., Chiu C.S., Konno R., Hutson P.H., Brandon N.J. Behavioral and biochemical characterization of a mutant mouse strain lacking D-amino acid oxidase activity and its implications for schizophrenia. Mol. Cell. Neurosci. 2006;32:324–334. - PubMed
-
- Andersen J.D., Pouzet B. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology. 2004;29:1080–1090. - PubMed
-
- Bauer D., Hamacher K., Bröer S., Pauleit D., Palm C., Zilles K., Coenen H.H., Langen K.J. Preferred stereoselective brain uptake of D-serine—a modulator of glutamatergic neurotransmission. Nucl. Med. Biol. 2005;32:793–797. - PubMed
-
- Ben Mamou C., Gamache K., Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat. Neurosci. 2006;9:1237–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
