The HLA-DRalpha chain is modified by polyubiquitination
- PMID: 19117940
- PMCID: PMC2652342
- DOI: 10.1074/jbc.M805736200
The HLA-DRalpha chain is modified by polyubiquitination
Abstract
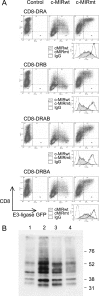
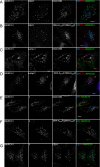
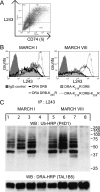
Ubiquitination plays a major role in regulating cell surface and intracellular localization of major histocompatibility complex class II molecules. Two E3 ligases, MARCH I and MARCH VIII, have been shown to polyubiquitinate lysine residue 225 in the cytoplasmic tail of I-Abeta and HLA-DRbeta. We show that lysine residue 219 in the cytoplasmic tail of DRalpha is also subject to polyubiquitination. Each chain of the HLA-DR heterodimer is independently recognized and ubiquitinated, but DRbeta is more extensively modified. In the cytoplasmic tail of DRbeta lysine, residue 225 is the only residue that is absolutely required for ubiquitination; all other residues can be deleted or substituted without loss of function. In contrast, although lysine 219 is absolutely required for modification of DRalpha, other features of the DRalpha tail act to limit the extent of ubiquitination.
Figures




References
-
- Peters, P. J., Neefjes, J. J., Oorschot, V., Ploegh, H. L., and Geuze, H. J. (1991) Nature 349 669-676 - PubMed
-
- Cresswell, P. (1992) Curr. Opin. Immunol. 4 87-92 - PubMed
-
- Denzin, L. K., and Cresswell, P. (1995) Cell 82 155-165 - PubMed
-
- Watts, C. (2004) Nat. Immunol. 5 685-692 - PubMed
-
- Hiltbold, E. M., and Roche, P. A. (2002) Curr. Opin. Immunol. 14 30-35 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

