Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts
- PMID: 19118022
- PMCID: PMC2613547
- DOI: 10.1158/0008-5472.CAN-08-2724
Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts
Abstract
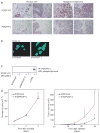
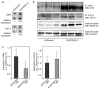
Cancer results from the concerted performance of malignant cells and stromal cells. Cell types populating the microenvironment are enlisted by the tumor to secrete a host of growth-promoting cues, thus upholding tumor initiation and progression. Platelet-derived growth factors (PDGF) support the formation of a prominent tumor stromal compartment by as of yet unidentified molecular effectors. Whereas PDGF-CC induces fibroblast reactivity and fibrosis in a range of tissues, little is known about the function of PDGF-CC in shaping the tumor-stroma interplay. Herein, we present evidence for a paracrine signaling network involving PDGF-CC and PDGF receptor-alpha in malignant melanoma. Expression of PDGFC in a mouse model accelerated tumor growth through recruitment and activation of different subsets of cancer-associated fibroblasts. In seeking the molecular identity of the supporting factors provided by cancer-associated fibroblasts, we made use of antibody arrays and an in vivo coinjection model to identify osteopontin as the effector of the augmented tumor growth induced by PDGF-CC. In conclusion, we establish paracrine signaling by PDGF-CC as a potential drug target to reduce stromal support in malignant melanoma.
Figures




References
-
- Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–20. - PubMed
-
- Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–97. - PubMed
-
- Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

