Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex
- PMID: 19118032
- PMCID: PMC4483278
- DOI: 10.1158/1078-0432.CCR-08-1805
Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex
Abstract
Purpose: Secretory clusterin (sCLU)/apolipoprotein J is an extracellular chaperone that has been functionally implicated in DNA repair, cell cycle regulation, apoptotic cell death, and tumorigenesis. It exerts a prosurvival function against most therapeutic treatments for cancer and is currently an antisense target in clinical trials for tumor therapy. However, the molecular mechanisms underlying its function remained largely unknown.
Experimental design: The molecular effects of small interfering RNA-mediated sCLU depletion in nonstressed human cancer cells were examined by focusing entirely on the endogenously expressed sCLU protein molecules and combining molecular, biochemical, and microscopic approaches.
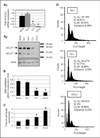
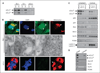
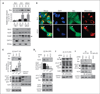
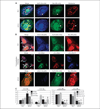
Results: We report here that sCLU depletion in nonstressed human cancer cells signals stress that induces p53-dependent growth retardation and high rates of endogenous apoptosis. We discovered that increased apoptosis in sCLU-depleted cells correlates to altered ratios of proapoptotic to antiapoptotic Bcl-2 protein family members, is amplified by p53, and is executed by mitochondrial dysfunction. sCLU depletion-related stress signals originate from several sites, because sCLU is an integral component of not only the secretory pathway but also the nucleocytosolic continuum and mitochondria. In the cytoplasm, sCLU depletion disrupts the Ku70-Bax complex and triggers Bax activation and relocation to mitochondria. We show that sCLU binds and thereby stabilizes the Ku70-Bax protein complex serving as a cytosol retention factor for Bax.
Conclusions: We suggest that elevated sCLU levels may enhance tumorigenesis by interfering with Bax proapoptotic activities and contribute to one of the major characteristics of cancer cells, that is, resistance to apoptosis.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29:5380–5389. - PubMed
-
- Wilson MR, Easterbrook-Smith SB. Clusterin is a secreted mammalian chaperone. Trends Biochem Sci. 2000;25:95–98. - PubMed
-
- Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34:1430–1448. - PubMed
-
- Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analysis of nuclear clusterin: a cell death protein. J Biol Chem. 2003;278:11590–11600. - PubMed
-
- Caccamo AE, Scaltriti M, Caporali A, et al. Ca2+ depletion induces nuclear clusterin, a novel effector of apoptosis in immortalized human prostate cells. Cell Death Differ. 2005;12:101–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

