A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3
- PMID: 19118066
- PMCID: PMC2865676
- DOI: 10.1158/1078-0432.CCR-08-1725
A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3
Abstract
Purpose: To evaluate the safety and immunogenicity of a therapeutic human papillomavirus (HPV)16 DNA vaccine administered to women with HPV16+cervical intraepithelial neoplasia (CIN)2/3.
Experimental design: This phase I trial incorporated the standard '3+3'' dose-escalation design with an additional 6 patients allocated to the maximally tolerated dose. Healthy adult women with colposcopically directed, biopsy-proven HPV16+ CIN2/3 received 3 i.m. vaccinations (0.5, 1, or 3 mg) of a plasmid expressing a Sig-E7(detox)-heat shock protein 70 fusion protein on days 0, 28, and 56, and underwent standard therapeutic resection of the cervical squamocolumnar junction at day 105 (week 15). The safety and immunogenicity of the vaccine and histologic outcome based on resection at week 15 were assessed.
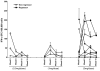
Results: Fifteen patients were evaluable (3 each at 0.5 and 1mg, 9 at 3 mg). The vaccine was well tolerated: most adverse events were mild, transient injection-site discomfort; no dose-limiting toxicities were observed. Although HPVE7-specific T-cell responses to E7 detected by enzyme-linked immunospot assays (IFN-gamma) were of low frequency and magnitude, detectable increases in response subsequent to vaccination were identified in subjects in the second and third cohorts. Complete histologic regression occurred in 3 of 9 (33%; 7-70% confidence interval) individuals in the highest-dose cohort. Although the difference is not significant, it is slightly higher than would be expected in an unvaccinated cohort (25%).
Conclusions: This HPV16 DNA vaccine was safe and well tolerated. Whereas it seems possible to elicit HPV-specific T-cell responses in patients with established dysplastic lesions, other factors are likely to play a role in lesion regression.
Trial registration: ClinicalTrials.gov NCT00121173.
Figures

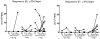
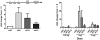
References
-
- Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60(4):1035–42. - PubMed
-
- Gravitt PE. Reproducibility of HPV16 and HPV18 viral load quantitation using TaqMan real-time PCR assays. J Virol Methods. 2003;112(1-2):23–33. - PubMed
-
- Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods. 2001;253(1-2):153–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

