Spred1 is required for synaptic plasticity and hippocampus-dependent learning
- PMID: 19118178
- PMCID: PMC6671253
- DOI: 10.1523/JNEUROSCI.4698-08.2008
Spred1 is required for synaptic plasticity and hippocampus-dependent learning
Abstract
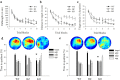
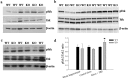
Germline mutations in SPRED1, a negative regulator of Ras, have been described in a neurofibromatosis type 1 (NF1)-like syndrome (NFLS) that included learning difficulties in some affected individuals. NFLS belongs to the group of phenotypically overlapping neuro-cardio-facial-cutaneous syndromes that are all caused by germ line mutations in genes of the Ras/mitogen-activated protein kinase extracellular signal-regulated kinase (ERK) pathway and that present with some degree of learning difficulties or mental retardation. We investigated hippocampus-dependent learning and memory as well as synaptic plasticity in Spred1(-/-) mice, an animal model of this newly discovered human syndrome. Spred1(-/-) mice show decreased learning and memory performance in the Morris water maze and visual-discrimination T-maze, but normal basic neuromotor and sensory abilities. Electrophysiological recordings on brain slices from these animals identified defects in short- and long-term synaptic hippocampal plasticity, including a disequilibrium between long-term potentiation (LTP) and long-term depression in CA1 region. Biochemical analysis, 4 h after LTP induction, demonstrated increased ERK-phosphorylation in Spred1(-/-) slices compared with those of wild-type littermates. This indicates that deficits in hippocampus-dependent learning and synaptic plasticity induced by SPRED1 deficiency are related to hyperactivation of the Ras/ERK pathway.
Figures


References
-
- AtkinsCM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD (1998) The ERK cascade is required for mammalian associative learning. Nat Neurosci 1:602–609. - PubMed
-
- Bentires-AljM, Kontaridis MI, Neel BG (2006) Stops along the RAS pathway in human genetic disease. Nat Med 12:283–285. - PubMed
-
- BremsH, Chmara M, Sahbatou M, Denayer E, Taniguchi K, Kato R, Somers R, Messiaen L, De Schepper S, Fryns JP, Cools J, Marynen P, Thomas G, Yoshimura A, Legius E (2007) Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet 39:1120–1126. - PubMed
-
- CasciT, Vinós J, Freeman M (1999) Sprouty, an intracellular inhibitor of Ras signaling. Cell 96:655–665. - PubMed
-
- CostaRM, Yang T, Huynh DP, Pulst SM, Viskochil DH, Silva AJ, Brannan CI (2001) Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet 27:399–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
