Combined targeting of lentiviral vectors and positioning of transduced cells by magnetic nanoparticles
- PMID: 19118196
- PMCID: PMC2629186
- DOI: 10.1073/pnas.0803746106
Combined targeting of lentiviral vectors and positioning of transduced cells by magnetic nanoparticles
Abstract
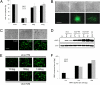
Targeting of viral vectors is a major challenge for in vivo gene delivery, especially after intravascular application. In addition, targeting of the endothelium itself would be of importance for gene-based therapies of vascular disease. Here, we used magnetic nanoparticles (MNPs) to combine cell transduction and positioning in the vascular system under clinically relevant, nonpermissive conditions, including hydrodynamic forces and hypothermia. The use of MNPs enhanced transduction efficiency of endothelial cells and enabled direct endothelial targeting of lentiviral vectors (LVs) by magnetic force, even in perfused vessels. In addition, application of external magnetic fields to mice significantly changed LV/MNP biodistribution in vivo. LV/MNP-transduced cells exhibited superparamagnetic behavior as measured by magnetorelaxometry, and they were efficiently retained by magnetic fields. The magnetic interactions were strong enough to position MNP-containing endothelial cells at the intima of vessels under physiological flow conditions. Importantly, magnetic positioning of MNP-labeled cells was also achieved in vivo in an injury model of the mouse carotid artery. Intravascular gene targeting can be combined with positioning of the transduced cells via nanomagnetic particles, thereby combining gene- and cell-based therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Verma IM, Weitzman MD. Gene therapy: Twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. - PubMed
-
- Wiznerowicz M, Trono D. Harnessing HIV for therapy, basic research and biotechnology. Trends Biotechnol. 2005;23:42–47. - PubMed
-
- Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

