Environmental tobacco smoke effects on lung surfactant film organization
- PMID: 19118518
- PMCID: PMC2637945
- DOI: 10.1016/j.bbamem.2008.11.021
Environmental tobacco smoke effects on lung surfactant film organization
Abstract
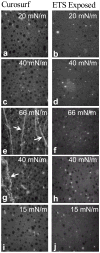
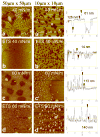
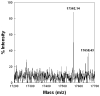
Adsorption of the clinical lung surfactants (LS) Curosurf or Survanta from aqueous suspension to the air-water interface progresses from multi-bilayer aggregates through multilayer films to a coexistence between multilayer and monolayer domains. Exposure to environmental tobacco smoke (ETS) alters this progression as shown by Langmuir isotherms, fluorescence microscopy and atomic force microscopy (AFM). After 12 h of LS exposure to ETS, AFM images of Langmuir-Blodgett deposited films show that ETS reduces the amount of material near the interface and alters how surfactant is removed from the interface during compression. For Curosurf, ETS prevents refining of the film composition during cycling; this leads to higher minimum surface tensions. ETS also changes the morphology of the Curosurf film by reducing the size of condensed phase domains from 8-12 microm to approximately 2 microm, suggesting a decrease in the line tension between the domains. The minimum surface tension and morphology of the Survanta film are less impacted by ETS exposure, although the amount of material associated with the film is reduced in a similar way to Curosurf. Fluorescence and mass spectra of Survanta dispersions containing native bovine SP-B treated with ETS indicate the oxidative degradation of protein aromatic amino acid residue side chains. Native bovine SP-C isolated from ETS exposed Survanta had changes in molecular mass consistent with deacylation of the lipoprotein. Fourier Transform Infrared Spectroscopy (FTIR) characterization of the hydrophobic proteins from ETS treated Survanta dispersions show significant changes in the conformation of SP-B and SP-C that correlate with the altered surface activity and morphology of the lipid-protein film.
Figures




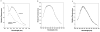
References
-
- Chan-Yeung M, Dimich-Ward H. Respiratory health effects of exposure to environmental tobacco smoke. Respirology. 2003;8:131–139. - PubMed
-
- Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH. Sidestream cigarette-smoke generation and exposure system for environmental tobacco-smoke studies. Inhalation Toxicology. 1994;6:79–93.
-
- Adlkofer F. Lung cancer due to passive smoking - A review. International Archives of Occupational and Environmental Health. 2001;74:231–241. - PubMed
-
- Dunn A, Zeine L. California Environmental Protection Agency. Sacramento, CA: 1997.
-
- Janson C. The effect of passive smoking on respiratory health in children and adults. International Journal of Tuberculosis and Lung Disease. 2004;8:510–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

