Age-associated changes in synaptic lipid raft proteins revealed by two-dimensional fluorescence difference gel electrophoresis
- PMID: 19118924
- PMCID: PMC2904836
- DOI: 10.1016/j.neurobiolaging.2008.11.005
Age-associated changes in synaptic lipid raft proteins revealed by two-dimensional fluorescence difference gel electrophoresis
Abstract
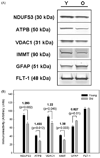
Brain aging is associated with a progressive decline in cognitive function though the molecular mechanisms remain unknown. Functional changes in brain neurons could be due to age-related alterations in levels of specific proteins critical for information processing. Specialized membrane microdomains known as 'lipid rafts' contain protein complexes involved in many signal transduction processes. This study was undertaken to determine if two-dimensional fluorescence difference gel electrophoresis (2D DIGE) analysis of proteins in synaptic membrane lipid rafts revealed age-dependent alterations in levels of raft proteins. Five pairs of young and aged rat synaptic membrane rafts were subjected to DIGE separation, followed by image analysis and identification of significantly altered proteins. Of 1046 matched spots on DIGE gels, 94 showed statistically significant differences in levels between old and young rafts, and 87 of these were decreased in aged rafts. The 41 most significantly altered (p<0.03) proteins included several synaptic proteins involved in energy metabolism, redox homeostasis, and cytoskeletal structure. This may indicate a disruption in bioenergetic balance and redox homeostasis in synaptic rafts with brain aging. Differential levels of representative identified proteins were confirmed by immunoblot analysis. Our findings provide novel pathways in investigations of mechanisms that may contribute to altered neuronal function in aging brain.
Copyright © 2008 Elsevier Inc. All rights reserved.
Conflict of interest statement
There is no conflict of interest.
Figures




Similar articles
-
Decreases in plasma membrane Ca²⁺-ATPase in brain synaptic membrane rafts from aged rats.J Neurochem. 2012 Dec;123(5):689-99. doi: 10.1111/j.1471-4159.2012.07918.x. Epub 2012 Oct 11. J Neurochem. 2012. PMID: 22889001 Free PMC article.
-
Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts.J Neurochem. 2001 Apr;77(1):1-12. doi: 10.1046/j.1471-4159.2001.t01-1-00132.x. J Neurochem. 2001. PMID: 11279256
-
Molecular and biophysical features of hippocampal "lipid rafts aging" are modified by dietary n-3 long-chain polyunsaturated fatty acids.Aging Cell. 2023 Aug;22(8):e13867. doi: 10.1111/acel.13867. Epub 2023 May 30. Aging Cell. 2023. PMID: 37254617 Free PMC article.
-
The isolation of detergent-resistant lipid rafts for two-dimensional electrophoresis.Methods Mol Biol. 2008;424:413-22. doi: 10.1007/978-1-60327-064-9_32. Methods Mol Biol. 2008. PMID: 18369879 Review.
-
Lipid rafts and neurodegeneration: structural and functional roles in physiologic aging and neurodegenerative diseases.J Lipid Res. 2020 May;61(5):636-654. doi: 10.1194/jlr.TR119000427. Epub 2019 Dec 23. J Lipid Res. 2020. PMID: 31871065 Free PMC article. Review.
Cited by
-
Quantitative profiling of brain lipid raft proteome in a mouse model of fragile X syndrome.PLoS One. 2015 Apr 7;10(4):e0121464. doi: 10.1371/journal.pone.0121464. eCollection 2015. PLoS One. 2015. PMID: 25849048 Free PMC article.
-
Citrulline diet supplementation improves specific age-related raft changes in wild-type rodent hippocampus.Age (Dordr). 2013 Oct;35(5):1589-606. doi: 10.1007/s11357-012-9462-2. Epub 2012 Aug 24. Age (Dordr). 2013. PMID: 22918749 Free PMC article.
-
Decreases in plasma membrane Ca²⁺-ATPase in brain synaptic membrane rafts from aged rats.J Neurochem. 2012 Dec;123(5):689-99. doi: 10.1111/j.1471-4159.2012.07918.x. Epub 2012 Oct 11. J Neurochem. 2012. PMID: 22889001 Free PMC article.
-
Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome.J Neurochem. 2010 Jun;113(6):1577-88. doi: 10.1111/j.1471-4159.2010.06719.x. Epub 2010 Mar 31. J Neurochem. 2010. PMID: 20374424 Free PMC article.
-
The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions.Front Aging Neurosci. 2011 May 23;3:8. doi: 10.3389/fnagi.2011.00008. eCollection 2011. Front Aging Neurosci. 2011. PMID: 21647399 Free PMC article.
References
-
- Alban A, David SO, Bjorkesten L, Andersson C, Sloge E, Lewis S, Currie I. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. - PubMed
-
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J. Neural. Transm. Suppl. 2000;59:133–154. - PubMed
-
- Bae TJ, Kim MS, Kim JW, Kim BW, Choo HJ, Lee JW, Kim KB, Lee CS, Kim JH, Chang SY, Kang CY, Lee SW, Ko YG. Lipid raft proteome reveals ATP synthase complex in the cell surface. Proteomics. 2004;4:3536–3548. - PubMed
-
- Baker MA, Lane DJ, Ly JD, De Pinto V, Lawen A. VDAC1 is a transplasma membrane NADH-ferricyanide reductase. J. Biol. Chem. 2004;279:4811–4819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

