Late developmental plasticity in the T helper 17 lineage
- PMID: 19119024
- PMCID: PMC3607320
- DOI: 10.1016/j.immuni.2008.11.005
Late developmental plasticity in the T helper 17 lineage
Abstract
Development of T helper (Th) 17 cells requires transforming growth factor (TGF)-beta and interleukin (IL)-6 and is independent of the Th1 pathway. Although T cells that produce interferon (IFN)-gamma are a recognized feature of Th17 cell responses, mice deficient for STAT4 and T-bet-two prototypical Th1 transcription factors-are protected from autoimmunity associated with Th17 pathogenesis. To examine the fate and pathogenic potential of Th17 cells and origin of IFN-gamma-producing T cells that emerge during Th17 immunity, we developed IL-17F reporter mice that identify cells committed to expression of IL-17F and IL-17A. Th17 cells required TGF-beta for sustained expression of IL-17F and IL-17A. In the absence of TGF-beta, both IL-23 and IL-12 acted to suppress IL-17 and enhance IFN-gamma production in a STAT4- and T-bet-dependent manner, albeit with distinct efficiencies. These results support a model of late Th17 developmental plasticity with implications for autoimmunity and host defense.
Figures
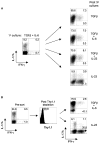






Comment in
-
Programming perpetual T helper cell plasticity.Immunity. 2009 Jan 16;30(1):7-9. doi: 10.1016/j.immuni.2008.12.012. Immunity. 2009. PMID: 19144312
References
-
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. - PubMed
-
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. - PubMed
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

