Cytosolic viral sensor RIG-I is a 5'-triphosphate-dependent translocase on double-stranded RNA
- PMID: 19119185
- PMCID: PMC3567915
- DOI: 10.1126/science.1168352
Cytosolic viral sensor RIG-I is a 5'-triphosphate-dependent translocase on double-stranded RNA
Abstract
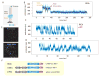
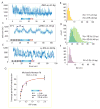
Retinoic acid inducible-gene I (RIG-I) is a cytosolic multidomain protein that detects viral RNA and elicits an antiviral immune response. Two N-terminal caspase activation and recruitment domains (CARDs) transmit the signal, and the regulatory domain prevents signaling in the absence of viral RNA. 5'-triphosphate and double-stranded RNA (dsRNA) are two molecular patterns that enable RIG-I to discriminate pathogenic from self-RNA. However, the function of the DExH box helicase domain that is also required for activity is less clear. Using single-molecule protein-induced fluorescence enhancement, we discovered a robust adenosine 5'-triphosphate-powered dsRNA translocation activity of RIG-I. The CARDs dramatically suppress translocation in the absence of 5'-triphosphate, and the activation by 5'-triphosphate triggers RIG-I to translocate preferentially on dsRNA in cis. This functional integration of two RNA molecular patterns may provide a means to specifically sense and counteract replicating viruses.
Figures


References
-
- Yoneyama M, et al. Nat Immunol. 2004 Jul;5:730. - PubMed
-
- Hornung V, et al. Science. 2006 Nov 10;314:994. - PubMed
-
- Pichlmair A, et al. Science. 2006 Nov 10;314:997. - PubMed
-
- Shatkin AJ, Manley JL. Nat Struct Biol. 2000 Oct;7:838. - PubMed
-
- Fromont-Racine M, Senger B, Saveanu C, Fasiolo F. Gene. 2003 Aug 14;313:17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

