Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants
- PMID: 19119321
- PMCID: PMC2606064
- DOI: 10.1371/journal.pone.0004096
Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants
Abstract
Background: Daily nevirapine (NVP) prophylaxis to HIV-exposed infants significantly reduces breast-milk HIV transmission. We assessed NVP-resistance in Indian infants enrolled in the "six-week extended-dose nevirapine" (SWEN) trial who received single-dose NVP (SD-NVP) or SWEN for prevention of breast-milk HIV transmission but who also acquired subtype C HIV infection during the first year of life.
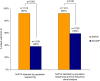
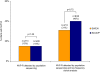
Methods/findings: Standard population sequencing and cloning for viral subpopulations present at > or =5% frequency were used to determine HIV genotypes from 94% of the 79 infected Indian infants studied. Timing of infection was defined based on when an infant's blood sample first tested positive for HIV DNA. SWEN-exposed infants diagnosed with HIV by six weeks of age had a significantly higher prevalence of NVP-resistance than those who received SD-NVP, by both standard population sequencing (92% of 12 vs. 38% of 29; p = 0.002) and low frequency clonal analysis (92% of 12 vs. 59% of 29; p = 0.06). Likelihood of infection with NVP-resistant HIV through breast-milk among infants infected after age six weeks was substantial, but prevalence of NVP-resistance did not differ among SWEN or SD-NVP exposed infants by standard population sequencing (15% of 13 vs. 15% of 20; p = 1.00) and clonal analysis (31% of 13 vs. 40% of 20; p = 0.72). Types of NVP-resistance mutations and patterns of persistence at one year of age were similar between the two groups. NVP-resistance mutations did differ by timing of HIV infection; the Y181C variant was predominant among infants diagnosed in the first six weeks of life, compared to Y188C/H during late breast-milk transmission.
Conclusions/significance: Use of SWEN to prevent breast-milk HIV transmission carries a high likelihood of resistance if infection occurs in the first six weeks of life. Moreover, there was a continued risk of transmission of NVP-resistant HIV through breastfeeding during the first year of life, but did not differ between SD-NVP and SWEN groups. As with SD-NVP, the value of preventing HIV infection in a large number of infants should be considered alongside the high risk of resistance associated with extended NVP prophylaxis.
Trial registration: ClinicalTrials.gov NCT00061321.
Conflict of interest statement
Figures
References
-
- UNAIDS and WHO. AIDS Epidemic Update. Geneva: UNAIDS. 2007. Available: www.unaids.org/en/HIV_data/2007EpiUpdate/default.asp. Accessed 24 September 2008.
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. - PubMed
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. - PubMed
-
- De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–1182. - PubMed
-
- Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

