Regulation of small RNA accumulation in the maize shoot apex
- PMID: 19119413
- PMCID: PMC2602737
- DOI: 10.1371/journal.pgen.1000320
Regulation of small RNA accumulation in the maize shoot apex
Abstract
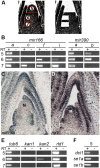
MicroRNAs (miRNAs) and trans-acting siRNAs (ta-siRNAs) are essential to the establishment of adaxial-abaxial (dorsoventral) leaf polarity. Tas3-derived ta-siRNAs define the adaxial side of the leaf by restricting the expression domain of miRNA miR166, which in turn demarcates the abaxial side of leaves by restricting the expression of adaxial determinants. To investigate the regulatory mechanisms that allow for the precise spatiotemporal accumulation of these polarizing small RNAs, we used laser-microdissection coupled to RT-PCR to determine the expression profiles of their precursor transcripts within the maize shoot apex. Our data reveal that the pattern of mature miR166 accumulation results, in part, from intricate transcriptional regulation of its precursor loci and that only a subset of mir166 family members contribute to the establishment of leaf polarity. We show that miR390, an upstream determinant in leaf polarity whose activity triggers tas3 ta-siRNA biogenesis, accumulates adaxially in leaves. The polar expression of miR390 is established and maintained independent of the ta-siRNA pathway. The comparison of small RNA localization data with the expression profiles of precursor transcripts suggests that miR166 and miR390 accumulation is also regulated at the level of biogenesis and/or stability. Furthermore, mir390 precursors accumulate exclusively within the epidermal layer of the incipient leaf, whereas mature miR390 accumulates in sub-epidermal layers as well. Regulation of miR390 biogenesis, stability, or even discrete trafficking of miR390 from the epidermis to underlying cell layers provide possible mechanisms that define the extent of miR390 accumulation within the incipient leaf, which patterns this small field of cells into adaxial and abaxial domains via the production of tas3-derived ta-siRNAs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



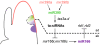
References
-
- Plasterk RH. MicroRNAs in animal development. Cell. 2006;124:877–881. - PubMed
-
- Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. - PubMed
-
- Chitwood DH, Guo M, Nogueira FTS, Timmermans MCP. Establishing leaf polarity: the role of small RNAs and positional signals in the shoot apex. Development. 2007;134:813–823. - PubMed
-
- McConnell J, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

