The cell adhesion molecule "CAR" and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution
- PMID: 19119424
- PMCID: PMC2607015
- DOI: 10.1371/journal.ppat.1000277
The cell adhesion molecule "CAR" and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution
Abstract
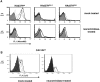
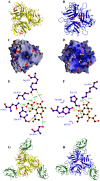
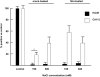
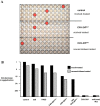
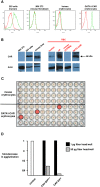
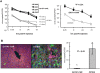
Although it has been known for 50 years that adenoviruses (Ads) interact with erythrocytes ex vivo, the molecular and structural basis for this interaction, which has been serendipitously exploited for diagnostic tests, is unknown. In this study, we characterized the interaction between erythrocytes and unrelated Ad serotypes, human 5 (HAd5) and 37 (HAd37), and canine 2 (CAV-2). While these serotypes agglutinate human erythrocytes, they use different receptors, have different tropisms and/or infect different species. Using molecular, biochemical, structural and transgenic animal-based analyses, we found that the primary erythrocyte interaction domain for HAd37 is its sialic acid binding site, while CAV-2 binding depends on at least three factors: electrostatic interactions, sialic acid binding and, unexpectedly, binding to the coxsackievirus and adenovirus receptor (CAR) on human erythrocytes. We show that the presence of CAR on erythrocytes leads to prolonged in vivo blood half-life and significantly reduced liver infection when a CAR-tropic Ad is injected intravenously. This study provides i) a molecular and structural rationale for Ad-erythrocyte interactions, ii) a basis to improve vector-mediated gene transfer and iii) a mechanism that may explain the biodistribution and pathogenic inconsistencies found between human and animal models.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Adenovirus coxsackie adenovirus receptor-mediated binding to human erythrocytes does not preclude systemic transduction.Cancer Gene Ther. 2016 Dec;23(12):411-414. doi: 10.1038/cgt.2016.50. Epub 2016 Oct 21. Cancer Gene Ther. 2016. PMID: 27767087
-
Adenovirus type 37 uses sialic acid as a cellular receptor.J Virol. 2000 Jan;74(1):42-8. J Virol. 2000. PMID: 10590089 Free PMC article.
-
Glycophorin-C sialylation regulates Lu/BCAM adhesive capacity during erythrocyte aging.Blood Adv. 2018 Jan 3;2(1):14-24. doi: 10.1182/bloodadvances.2017013094. eCollection 2018 Jan 9. Blood Adv. 2018. PMID: 29344581 Free PMC article.
-
Sialic acid binding receptors (siglecs) expressed by macrophages.J Leukoc Biol. 1999 Nov;66(5):705-11. doi: 10.1002/jlb.66.5.705. J Leukoc Biol. 1999. PMID: 10577497 Review.
-
New functions for the sialic acid-binding adhesion molecule CD22, a member of the growing family of Siglecs.Scand J Immunol. 2001 Mar;53(3):227-34. doi: 10.1046/j.1365-3083.2001.00868.x. Scand J Immunol. 2001. PMID: 11251878 Review. No abstract available.
Cited by
-
Integrin alphavbeta5 is a primary receptor for adenovirus in CAR-negative cells.Virol J. 2010 Jul 8;7:148. doi: 10.1186/1743-422X-7-148. Virol J. 2010. PMID: 20615244 Free PMC article.
-
Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors.Blood. 2010 Oct 14;116(15):2656-64. doi: 10.1182/blood-2009-12-260026. Epub 2010 Jul 7. Blood. 2010. PMID: 20610817 Free PMC article.
-
A Siglec-like sialic-acid-binding motif revealed in an adenovirus capsid protein.Glycobiology. 2012 Aug;22(8):1086-91. doi: 10.1093/glycob/cws073. Epub 2012 Apr 21. Glycobiology. 2012. PMID: 22522600 Free PMC article.
-
Structural dynamics of SARS-CoV-2 variants: A health monitoring strategy for anticipating Covid-19 outbreaks.J Infect. 2021 Aug;83(2):197-206. doi: 10.1016/j.jinf.2021.06.001. Epub 2021 Jun 3. J Infect. 2021. PMID: 34089757 Free PMC article.
-
Human coagulation factor X-adenovirus type 5 complexes poorly stimulate an innate immune response in human mononuclear phagocytes.J Virol. 2015 Mar;89(5):2884-91. doi: 10.1128/JVI.03576-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540380 Free PMC article.
References
-
- Cusack S. Adenovirus complex structures. Curr Opin Struct Biol. 2005;15:237–243. - PubMed
-
- Gelderblom H, Maichle-Lauppe I. The fibers of fowl adenoviruses. Arch Virol. 1982;72:289–298. - PubMed
-
- Albinsson B, Kidd AH. Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 1999;64:125–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

