Small-molecule peptides inhibit Z alpha1-antitrypsin polymerization
- PMID: 19120695
- PMCID: PMC6529975
- DOI: 10.1111/j.1582-4934.2008.00608.x
Small-molecule peptides inhibit Z alpha1-antitrypsin polymerization
Abstract
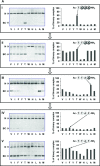
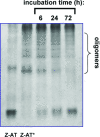
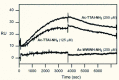
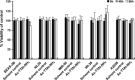
The Z variant of 1-antitrypsin (AT) polymerizes within the liver and gives rise to liver cirrhosis and the associated plasma deficiency leads to emphysema. In this work, a combinatorial approach based on the inhibitory mechanism of (alpha1)-AT was developed to arrest its pathogenic polymerization. One peptide, Ac-TTAI-NH(2), emerged as the most tight-binding ligand for Z (alpha1)-AT. Characterization of this tetrapeptide by gel electrophoresis and biosensor analysis revealed its markedly improved binding specificity and affinity compared with all previously reported peptide inhibitors. In addition, the peptide is not cytotoxic to lung cell lines. A model of the peptide-protein complex suggests that the peptide interacts with nearby residues by hydrogen bonds, hydrophobic interactions, and cavity-filling stabilization. The combinatorially selected peptide not only effectively blocks the polymerization but also promotes dissociation of the oligomerized (alpha1)-AT. These results are a significant step towards the potential treatment of Z (alpha1)-AT related diseases.
Figures



Similar articles
-
Identification of a 4-mer peptide inhibitor that effectively blocks the polymerization of pathogenic Z alpha1-antitrypsin.Am J Respir Cell Mol Biol. 2006 Nov;35(5):540-8. doi: 10.1165/rcmb.2005-0207OC. Epub 2006 Jun 15. Am J Respir Cell Mol Biol. 2006. PMID: 16778151
-
Molecular Mechanism of Z α1-Antitrypsin Deficiency.J Biol Chem. 2016 Jul 22;291(30):15674-86. doi: 10.1074/jbc.M116.727826. Epub 2016 May 31. J Biol Chem. 2016. PMID: 27246852 Free PMC article.
-
Prevention of polymerization of M and Z alpha1-Antitrypsin (alpha1-AT) with trimethylamine N-oxide. Implications for the treatment of alpha1-at deficiency.Am J Respir Cell Mol Biol. 2001 Jun;24(6):727-32. doi: 10.1165/ajrcmb.24.6.4407. Am J Respir Cell Mol Biol. 2001. PMID: 11415938
-
Loop-sheet polymerization: the structural basis of Z alpha 1-antitrypsin accumulation in the liver.Clin Sci (Lond). 1994 May;86(5):489-95. doi: 10.1042/cs0860489. Clin Sci (Lond). 1994. PMID: 8033502 Review.
-
Genetic variants of alpha1-antitrypsin.Curr Protein Pept Sci. 2010 Mar;11(2):101-17. doi: 10.2174/138920310790848368. Curr Protein Pept Sci. 2010. PMID: 19751191 Review.
Cited by
-
Targeting the VEGF-C/VEGFR3 axis suppresses Slug-mediated cancer metastasis and stemness via inhibition of KRAS/YAP1 signaling.Oncotarget. 2017 Jan 17;8(3):5603-5618. doi: 10.18632/oncotarget.13629. Oncotarget. 2017. PMID: 27901498 Free PMC article. Review.
-
Defining the mechanism of polymerization in the serpinopathies.Proc Natl Acad Sci U S A. 2010 Oct 5;107(40):17146-51. doi: 10.1073/pnas.1004785107. Epub 2010 Sep 20. Proc Natl Acad Sci U S A. 2010. PMID: 20855577 Free PMC article.
-
Impact of the PEG length and PEGylation site on the structural, thermodynamic, thermal, and proteolytic stability of mono-PEGylated alpha-1 antitrypsin.Protein Sci. 2022 Sep;31(9):e4392. doi: 10.1002/pro.4392. Protein Sci. 2022. PMID: 36040264 Free PMC article.
-
Z α1-antitrypsin confers a proinflammatory phenotype that contributes to chronic obstructive pulmonary disease.Am J Respir Crit Care Med. 2014 Apr 15;189(8):909-31. doi: 10.1164/rccm.201308-1458OC. Am J Respir Crit Care Med. 2014. PMID: 24592811 Free PMC article.
-
Z α-1 antitrypsin deficiency and the endoplasmic reticulum stress response.World J Gastrointest Pharmacol Ther. 2010 Oct 6;1(5):94-101. doi: 10.4292/wjgpt.v1.i5.94. World J Gastrointest Pharmacol Ther. 2010. PMID: 21577302 Free PMC article.
References
-
- Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha‐1‐antitrypsin deficiency. Am J Med. 1988; 84: 13–31. - PubMed
-
- Elliott PR, Lomas DA, Carrell RW, et al . Inhibitory conformation of the reactive loop of α1‐antitrypsin. Nat Struct Biol. 1996; 3: 676–81. - PubMed
-
- Loebermann H, Tokuoka R, Deisenhofer J, et al . Human α1‐proteinase inhibitor: crystal structure analysis of two crystal modifications, molecular model, and preliminary analysis of the implications for function. J Mol Biol. 1984; 177: 531–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

