Depression is an early disease manifestation in lupus-prone MRL/lpr mice
- PMID: 19121871
- PMCID: PMC2675630
- DOI: 10.1016/j.jneuroim.2008.11.009
Depression is an early disease manifestation in lupus-prone MRL/lpr mice
Abstract
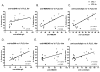
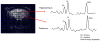
Many lupus patients develop neuropsychiatric manifestations, including cognitive dysfunction, depression, and anxiety. However, it is not clear if neuropsychiatric lupus is a primary disease manifestation, or is secondary to non-CNS disease. We found that MRL/lpr lupus-prone mice exhibited significant depression-like behavior already at 8 weeks of age, despite normal visual working memory, locomotor coordination and social preference. Moreover, depression was significantly correlated with titers of autoantibodies against DNA, NMDA receptors and cardiolipin. Our results indicate that lupus mice develop depression and CNS dysfunction very early in the course of disease, in the absence of substantial pathology involving other target organs.
Figures




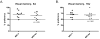




References
-
- Bessa JM, Oliveira M, Cerqueira JJ, Almeida OF, Sousa N. Age-related qualitative shift in emotional behaviour: paradoxical findings after re-exposure of rats in the elevated-plus maze. Behav Brain Res. 2005;162:135–142. - PubMed
-
- Bluestein HG. The central nervous system in systemic lupus erythematosus. In: Lahita RG, editor. Systemic lupus erythematosus. Churchill Livingstone; New York: 1992. pp. 639–655.
-
- Bonfa E, Marshak-Rothstein A, Weissbach H, Brot N, Elkon K. Frequency and epitope recognition of anti-ribosome P antibodies from humans with systemic lupus erythematosus and MRL/lpr mice are similar. J Immunol. 1988;140:3434–3437. - PubMed
-
- Brey RL, Abbott RD, Curb JD, Sharp DS, Ross GW, Stallworth CL, Kittner SJ. beta(2)-Glycoprotein 1-dependent anticardiolipin antibodies and risk of ischemic stroke and myocardial infarction: the honolulu heart program. Stroke. 2001;32:1701–1706. - PubMed
-
- Brooks WM, Sabet A, Sibbitt WL, Jr, Barker PB, van Zijl PC, Duyn JH, Moonen CT. Neurochemistry of brain lesions determined by spectroscopic imaging in systemic lupus erythematosus. J Rheumatol. 1997;24:2323–2329. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

