Selective serotonin reuptake inhibitor use and risk of gestational hypertension
- PMID: 19122006
- PMCID: PMC2735348
- DOI: 10.1176/appi.ajp.2008.08060817
Selective serotonin reuptake inhibitor use and risk of gestational hypertension
Abstract
Objective: The purpose of this study was to assess the effects of treatment with selective serotonin reuptake inhibitors (SSRIs) on the risks of gestational hypertension and preeclampsia.
Method: The authors analyzed data from 5,731 women with nonmalformed infants and no underlying hypertension who participated in the Slone Epidemiology Center Birth Defects Study from 1998 to 2007. Gestational hypertension was defined as incident hypertension diagnosed after 20 weeks of pregnancy, with and without proteinuria (i.e., with and without preeclampsia). The risks of gestational hypertension and preeclampsia were compared between women who did and did not receive SSRI treatment during pregnancy. Relative risks and 95% confidence intervals (CIs) were estimated using the Cox proportional hazards model, adjusting for prepregnancy sociodemographic, lifestyle, reproductive, and medical factors.
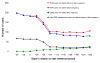
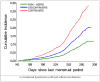
Results: Gestational hypertension was present in 9.0% of the 5,532 women who were not treated with SSRIs and 19.1% of the 199 women who were treated with SSRIs. Among women who received treatment, gestational hypertension was present in 13.1% of the 107 women who received treatment only during the first trimester and in 26.1% of the 92 women who continued treatment beyond the first trimester. The occurrence of preeclampsia was 2.4% among women who were not treated with SSRIs, 3.7% among women who were exposed to SSRIs only during the first trimester, and 15.2% among women who continued SSRI treatment beyond the first trimester. Relative to women who did not receive treatment, the adjusted relative risk of preeclampsia was 1.4 for women who discontinued treatment and 4.9 for women who continued treatment.
Conclusion: SSRI exposure during late pregnancy-whether a causal factor or not-might identify women who are at an increased risk for gestational hypertension and preeclampsia. Further investigation is needed in order to separate the effects of treatment with SSRIs from those of underlying mood disorders.
Conflict of interest statement
Figures

Comment in
-
Parsing risk for the use of selective serotonin reuptake inhibitors in pregnancy.Am J Psychiatry. 2009 Mar;166(3):268-70. doi: 10.1176/appi.ajp.2008.08111703. Am J Psychiatry. 2009. PMID: 19255046 Free PMC article. No abstract available.
Similar articles
-
Prenatal Selective Serotonin Reuptake Inhibitor Use and Associated Risk for Gestational Hypertension and Preeclampsia: A Meta-Analysis of Cohort Studies.J Womens Health (Larchmt). 2018 Jun;27(6):791-800. doi: 10.1089/jwh.2017.6642. Epub 2018 Feb 28. J Womens Health (Larchmt). 2018. PMID: 29489446
-
Risk of preeclampsia after gestational exposure to selective serotonin reuptake inhibitors and other antidepressants: A study from The Norwegian Mother and Child Cohort Study.Pharmacoepidemiol Drug Saf. 2017 Oct;26(10):1266-1276. doi: 10.1002/pds.4286. Epub 2017 Aug 16. Pharmacoepidemiol Drug Saf. 2017. PMID: 28815791 Free PMC article.
-
Selective serotonin reuptake inhibitors and preeclampsia: A quality assessment and meta-analysis.Pregnancy Hypertens. 2022 Dec;30:36-43. doi: 10.1016/j.preghy.2022.08.001. Epub 2022 Aug 6. Pregnancy Hypertens. 2022. PMID: 35963154 Free PMC article. Review.
-
[Treatment of depressed pregnant women by selective serotonin reuptake inhibitors: risk for the foetus and the newborn].Encephale. 2010 Jun;36 Suppl 2:D133-8. doi: 10.1016/j.encep.2009.06.005. Epub 2009 Sep 19. Encephale. 2010. PMID: 20513456 Review. French.
-
Risk of gestational hypertension and preeclampsia in women who discontinued or continued antidepressant medication use during pregnancy.Arch Womens Ment Health. 2016 Dec;19(6):1051-1061. doi: 10.1007/s00737-016-0655-z. Epub 2016 Aug 24. Arch Womens Ment Health. 2016. PMID: 27558246
Cited by
-
Antidepressant use and gestational hypertension: does evidence support causality?Br J Clin Pharmacol. 2013 May;75(5):1373-4. doi: 10.1111/j.1365-2125.2012.04468.x. Br J Clin Pharmacol. 2013. PMID: 22994305 Free PMC article. No abstract available.
-
Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants?Am J Epidemiol. 2012 May 15;175(10):988-97. doi: 10.1093/aje/kwr394. Epub 2012 Mar 22. Am J Epidemiol. 2012. PMID: 22442287 Free PMC article.
-
Antidepressant Drugs Effects on Blood Pressure.Front Cardiovasc Med. 2021 Aug 3;8:704281. doi: 10.3389/fcvm.2021.704281. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34414219 Free PMC article. Review.
-
Dopamine in the Pathophysiology of Preeclampsia and Gestational Hypertension: Monoamine Oxidase (MAO) and Catechol-O-methyl Transferase (COMT) as Possible Mechanisms.Oxid Med Cell Longev. 2019 Nov 28;2019:3546294. doi: 10.1155/2019/3546294. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31871546 Free PMC article. Review.
-
Antidepressant prescriptions, discontinuation, depression and perinatal outcomes, including breastfeeding: A population cohort analysis.PLoS One. 2019 Nov 18;14(11):e0225133. doi: 10.1371/journal.pone.0225133. eCollection 2019. PLoS One. 2019. PMID: 31738813 Free PMC article.
References
-
- Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269–74. - PubMed
-
- Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544 e1–5. - PubMed
-
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, Staffa JA, Platt R. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194 e1–5. - PubMed
-
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

