A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex
- PMID: 19122103
- PMCID: PMC2648091
- DOI: 10.1105/tpc.108.061317
A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex
Abstract
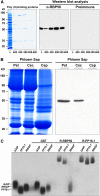
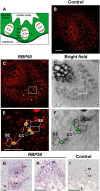
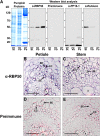
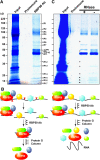
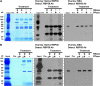
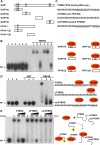
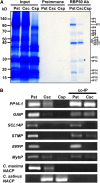
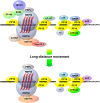
RNA binding proteins (RBPs) are integral components of ribonucleoprotein (RNP) complexes and play a central role in RNA processing. In plants, some RBPs function in a non-cell-autonomous manner. The angiosperm phloem translocation stream contains a unique population of RBPs, but little is known regarding the nature of the proteins and mRNA species that constitute phloem-mobile RNP complexes. Here, we identified and characterized a 50-kD pumpkin (Cucurbita maxima cv Big Max) phloem RNA binding protein (RBP50) that is evolutionarily related to animal polypyrimidine tract binding proteins. In situ hybridization studies indicated a high level of RBP50 transcripts in companion cells, while immunolocalization experiments detected RBP50 in both companion cells and sieve elements. A comparison of the levels of RBP50 present in vascular bundles and phloem sap indicated that this protein is highly enriched in the phloem sap. Heterografting experiments confirmed that RBP50 is translocated from source to sink tissues. Collectively, these findings established that RBP50 functions as a non-cell-autonomous RBP. Protein overlay, coimmunoprecipitation, and cross-linking experiments identified the phloem proteins and mRNA species that constitute RBP50-based RNP complexes. Gel mobility-shift assays demonstrated that specificity, with respect to the bound mRNA, is established by the polypyrimidine tract binding motifs within such transcripts. We present a model for RBP50-based RNP complexes within the pumpkin phloem translocation stream.
Figures

Similar articles
-
CmRBP50 protein phosphorylation is essential for assembly of a stable phloem-mobile high-affinity ribonucleoprotein complex.J Biol Chem. 2011 Jul 1;286(26):23142-9. doi: 10.1074/jbc.M111.244129. Epub 2011 May 13. J Biol Chem. 2011. PMID: 21572046 Free PMC article.
-
PbTTG1 forms a ribonucleoprotein complex with polypyrimidine tract-binding protein PbPTB3 to facilitate the long-distance trafficking of PbWoxT1 mRNA.Plant Sci. 2019 Mar;280:424-432. doi: 10.1016/j.plantsci.2019.01.008. Epub 2019 Jan 16. Plant Sci. 2019. PMID: 30824022
-
Pumpkin eIF5A isoforms interact with components of the translational machinery in the cucurbit sieve tube system.Plant J. 2010 Nov;64(3):536-50. doi: 10.1111/j.1365-313X.2010.04347.x. Epub 2010 Oct 1. Plant J. 2010. PMID: 20807213
-
Phloem-Mobile RNAs as Systemic Signaling Agents.Annu Rev Plant Biol. 2017 Apr 28;68:173-195. doi: 10.1146/annurev-arplant-042916-041139. Epub 2017 Jan 11. Annu Rev Plant Biol. 2017. PMID: 28125282 Review.
-
Mobile Transcripts and Intercellular Communication in Plants.Enzymes. 2016;40:1-29. doi: 10.1016/bs.enz.2016.07.001. Epub 2016 Aug 29. Enzymes. 2016. PMID: 27776778 Review.
Cited by
-
The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions.Nat Genet. 2013 Jan;45(1):51-8. doi: 10.1038/ng.2470. Epub 2012 Nov 25. Nat Genet. 2013. PMID: 23179023
-
New aspects of Phloem-mediated long-distance lipid signaling in plants.Front Plant Sci. 2012 Mar 28;3:53. doi: 10.3389/fpls.2012.00053. eCollection 2012. Front Plant Sci. 2012. PMID: 22639651 Free PMC article.
-
mRNA Localization in Plant Cells.Plant Physiol. 2020 Jan;182(1):97-109. doi: 10.1104/pp.19.00972. Epub 2019 Oct 14. Plant Physiol. 2020. PMID: 31611420 Free PMC article. Review.
-
A three-dimensional RNA motif in Potato spindle tuber viroid mediates trafficking from palisade mesophyll to spongy mesophyll in Nicotiana benthamiana.Plant Cell. 2011 Jan;23(1):258-72. doi: 10.1105/tpc.110.081414. Epub 2011 Jan 21. Plant Cell. 2011. PMID: 21258006 Free PMC article.
-
Long distance signalling and epigenetic changes in crop grafting.Front Plant Sci. 2023 Mar 20;14:1121704. doi: 10.3389/fpls.2023.1121704. eCollection 2023. Front Plant Sci. 2023. PMID: 37021313 Free PMC article. Review.
References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

