Peptide-based Biopolymers in Biomedicine and Biotechnology
- PMID: 19122836
- PMCID: PMC2575411
- DOI: 10.1016/j.mser.2008.04.004
Peptide-based Biopolymers in Biomedicine and Biotechnology
Abstract
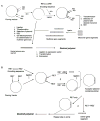
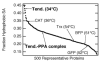
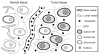
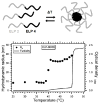
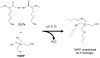
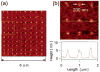
Peptides are emerging as a new class of biomaterials due to their unique chemical, physical, and biological properties. The development of peptide-based biomaterials is driven by the convergence of protein engineering and macromolecular self-assembly. This review covers the basic principles, applications, and prospects of peptide-based biomaterials. We focus on both chemically synthesized and genetically encoded peptides, including poly-amino acids, elastin-like polypeptides, silk-like polymers and other biopolymers based on repetitive peptide motifs. Applications of these engineered biomolecules in protein purification, controlled drug delivery, tissue engineering, and biosurface engineering are discussed.
Figures















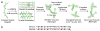



References
-
- Burkhard P, Stetefeld J, Strelkov SV. Trends In Cell Biology. 2001;11:82. - PubMed
-
- DeGrado WF, Summa CM, Pavone V, Nastri F, Lombardi A. Annual Review Of Biochemistry. 1999;68:779. - PubMed
-
- Debelle L, Tamburro AM. International Journal Of Biochemistry & Cell Biology. 1999;31:261. - PubMed
-
- Iozzo RV. Annual Review Of Biochemistry. 1998;67:609. - PubMed
-
- Bryson JW, Betz SF, Lu HS, Suich DJ, Zhou HXX, Oneil KT, Degrado WF. Science. 1995;270:935. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
