A Colorimetric Chemodosimeter for Pd(II): A Method for Detecting Residual Palladium in Cross-Coupling Reactions
- PMID: 19122841
- PMCID: PMC2583114
- DOI: 10.1016/j.tet.2008.04.105
A Colorimetric Chemodosimeter for Pd(II): A Method for Detecting Residual Palladium in Cross-Coupling Reactions
Abstract
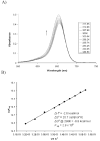
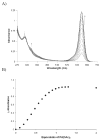
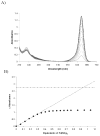
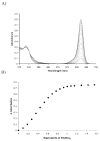
A colorimetric chemodosimeter (SQ1) for the detection of trace palladium salts in cross-coupling reactions mediated by palladium is described. Decolorization of SQ1 is affected by nucleophilic attack of ethanethiol in basic DMSO solutions. Thiol addition is determined to have an equilibrium constant (K(eq)) of 2.9 × 10(6) M(-1), with a large entropic and modest enthalpic driving force. This unusual result is attributed to solvent effects arising from a strong coordinative interaction between DMSO and the parent squaraine. Palladium detection is achieved through thiol scavenging from the SQ1-ethanethiol complex leading to a color "turn-on" of the parent squaraine. It was found that untreated samples obtained directly from Suzuki couplings showed no response to the assay. However, treatment of the samples with aqueous nitric acid generates a uniform Pd(NO(3))(2) species, which gives an appropriate response. "Naked-eye" detection of Pd(NO(3))(2) was estimated to be as low as 0.5 ppm in solution, and instrument-based detection was tested as low as 100 ppb. The average error over the working range of the assay was determined to be 7%.
Figures





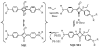

References
-
- Liesen PJ, Varma RS, Naicker KP. Tetrahedron Lett. 1999;40:2075–2078.
-
- Liu W-J, Xie Y-X, Liang Y, Li J-H. Synthesis. 2006:860–864.
-
- Baxter JM, Steinhuebel D, Palucki M, Davies IW. Org Lett. 2005;7:215–218. - PubMed
-
- Buchwald SL, Mauger C, Mignani G, Scholzc U. Adv Synth Catal. 2006;348:23–39.
-
- Arvela Riina K, Leadbeater NE, Collins Michael J., J Tetrahedron. 2005;61:9349–9355.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
