Conivaptan bolus dosing for the correction of hyponatremia in the neurointensive care unit
- PMID: 19123060
- PMCID: PMC2820273
- DOI: 10.1007/s12028-008-9179-3
Conivaptan bolus dosing for the correction of hyponatremia in the neurointensive care unit
Abstract
Introduction: Hyponatremia frequently complicates acute brain injury and may precipitate neurological worsening by promoting cerebral edema. An increase in brain water may be better managed through water excretion than with fluid restriction or hypertonic fluids. Vasopressin-receptor antagonists such as conivaptan, which promote free water excretion, may be ideal agents to treat this common and potentially serious disorder.
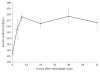
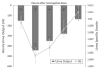
Methods: The efficacy of intermittent bolus doses of conivaptan to correct hyponatremia was examined in a consecutive series of patients treated in our neurointensive care unit. Patients were excluded if baseline sodium was over 135 mEq/l or if another conivaptan dose was given within 12 h. We assessed the proportion responding with a 4 or 6 mEq/l rise in sodium by 12 h, the change in sodium from baseline, and, in those not receiving another dose for at least 72 h, the long-term ability of a single dose to maintain sodium at least 4 mEq/l above baseline. We also recorded the effects of conivaptan on urine output and specific gravity, and noted any adverse events.
Results: A total of 25 doses given to 19 patients were included (out of 44 total doses administered in the study period). Sodium rose by 5.8 +/- 3.2 mEq/l within 12 h, with 71% rising by at least 4 mEq/l and 52% manifesting at least a 6 mEq/l increase. In those receiving only a single dose, 69% maintained at least a 4 mEq/l rise up to 72 h. Conivaptan also consistently led to increased urine output and a significant drop in urine specific gravity (i.e., aquaresis). No cases of phlebitis were observed despite administration of conivaptan through peripheral IVs.
Conclusion: Intermittent dosing of conivaptan was effective in increasing free water excretion and correcting hyponatremia in neurologically ill patients. This supports its further evaluation for managing hyponatremia in this population.
Figures
Similar articles
-
Conivaptan for hyponatremia in the neurocritical care unit.Neurocrit Care. 2009;11(1):6-13. doi: 10.1007/s12028-008-9152-1. Epub 2008 Nov 12. Neurocrit Care. 2009. PMID: 19003543
-
Effectiveness and Tolerability of Conivaptan and Tolvaptan for the Treatment of Hyponatremia in Neurocritically Ill Patients.Pharmacotherapy. 2017 May;37(5):528-534. doi: 10.1002/phar.1926. Epub 2017 Apr 17. Pharmacotherapy. 2017. PMID: 28295447
-
Efficacy and safety of oral conivaptan: a V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia.J Clin Endocrinol Metab. 2006 Jun;91(6):2145-52. doi: 10.1210/jc.2005-2287. Epub 2006 Mar 7. J Clin Endocrinol Metab. 2006. PMID: 16522696 Clinical Trial.
-
Conivaptan: new treatment for hyponatremia.Am J Health Syst Pharm. 2007 Jul 1;64(13):1385-95. doi: 10.2146/ajhp060383. Am J Health Syst Pharm. 2007. PMID: 17592003 Review.
-
[Vasopressin antagonists in treatment of hyponatremia].Pol Arch Med Wewn. 2007 Aug;117(8):356-62. Pol Arch Med Wewn. 2007. PMID: 18018383 Review. Polish.
Cited by
-
Continuous IV Infusion is the Choice Treatment Route for Arginine-vasopressin Receptor Blocker Conivaptan in Mice to Study Stroke-evoked Brain Edema.J Vis Exp. 2016 Sep 1;(115):54170. doi: 10.3791/54170. J Vis Exp. 2016. PMID: 27684044 Free PMC article.
-
Vasopressin receptor antagonists.Curr Hypertens Rep. 2015 Jan;17(1):510. doi: 10.1007/s11906-014-0510-4. Curr Hypertens Rep. 2015. PMID: 25604388 Review.
-
Brain edema formation and therapy after intracerebral hemorrhage.Neurobiol Dis. 2023 Jan;176:105948. doi: 10.1016/j.nbd.2022.105948. Epub 2022 Dec 5. Neurobiol Dis. 2023. PMID: 36481437 Free PMC article. Review.
-
Conivaptan for the Reduction of Cerebral Edema in Intracerebral Hemorrhage: A Safety and Tolerability Study.Clin Drug Investig. 2020 May;40(5):503-509. doi: 10.1007/s40261-020-00911-9. Clin Drug Investig. 2020. PMID: 32253717 Clinical Trial.
-
Urea for treatment of acute SIADH in patients with subarachnoid hemorrhage: a single-center experience.Ann Intensive Care. 2012 May 30;2(1):13. doi: 10.1186/2110-5820-2-13. Ann Intensive Care. 2012. PMID: 22647340 Free PMC article.
References
-
- Doczi T, Tarjanyi J, Huszka E, Kiss J. Syndrome of inappropriate secretion of antidiuretic hormone after head injury. Neurosurgery. 1982;10(6 Pt 1):685–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical