Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing
- PMID: 19123916
- PMCID: PMC3276350
- DOI: 10.1359/jbmr.090101
Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing
Abstract
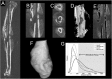
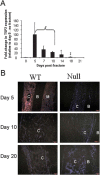
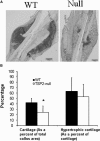
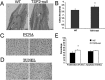
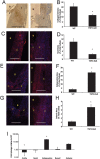
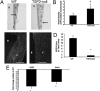
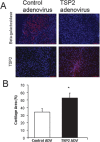
Thrombospondin-2 (TSP2) is a matricellular protein with increased expression during growth and regeneration. TSP2-null mice show accelerated dermal wound healing and enhanced bone formation. We hypothesized that bone regeneration would be enhanced in the absence of TSP2. Closed, semistabilized transverse fractures were created in the tibias of wildtype (WT) and TSP2-null mice. The fractures were examined 5, 10, and 20 days after fracture using microCT, histology, immunohistochemistry, quantitative RT-PCR, and torsional mechanical testing. Ten days after fracture, TSP2-null mice showed 30% more bone by microCT and 40% less cartilage by histology. Twenty days after fracture, TSP2-null mice showed reduced bone volume fraction and BMD. Mice were examined 5 days after fracture during the stage of neovascularization and mesenchymal cell influx to determine a cellular explanation for the phenotype. TSP2-null mice showed increased cell proliferation with no difference in apoptosis in the highly cellular fracture callus. Although mature bone and cartilage is minimal 5 days after fracture, TSP2-null mice had reduced expression of collagen IIa and Sox9 (chondrocyte differentiation markers) but increased expression of osteocalcin and osterix (osteoblast differentiation markers). Importantly, TSP2-null mice had a 2-fold increase in vessel density that corresponded with a reduction in vascular endothelial growth factor (VEGF) and Glut-1 (markers of hypoxia inducible factor [HIF]-regulated transcription). Finally, by expressing TSP2 using adenovirus starting 3 days after fracture, chondrogenesis was restored in TSP2-null mice. We hypothesize that TSP2 expressed by cells in the fracture mesenchyme regulates callus vascularization. The increase in vascularity increases tissue oxemia and decreases HIF; thus, undifferentiated cells in the callus develop into osteoblasts rather than chondrocytes. This leads to an alternative strategy for achieving fracture healing with reduced endochondral ossification and enhanced appositional bone formation. Controlling the ratio of cartilage to bone during fracture healing has important implications for expediting healing or promoting regeneration in nonunions.
Figures
References
-
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;355(Suppl):S7–S21. - PubMed
-
- Li G, White G, Connolly C, Marsh D. Cell proliferation and apoptosis during fracture healing. J Bone Miner Res. 2002;17:791–799. - PubMed
-
- Ford JL, Robinson DE, Scammell BE. The fate of soft callus chondrocytes during long bone fracture repair. J Orthop Res. 2003;21:54–61. - PubMed
-
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. - PubMed
-
- Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

